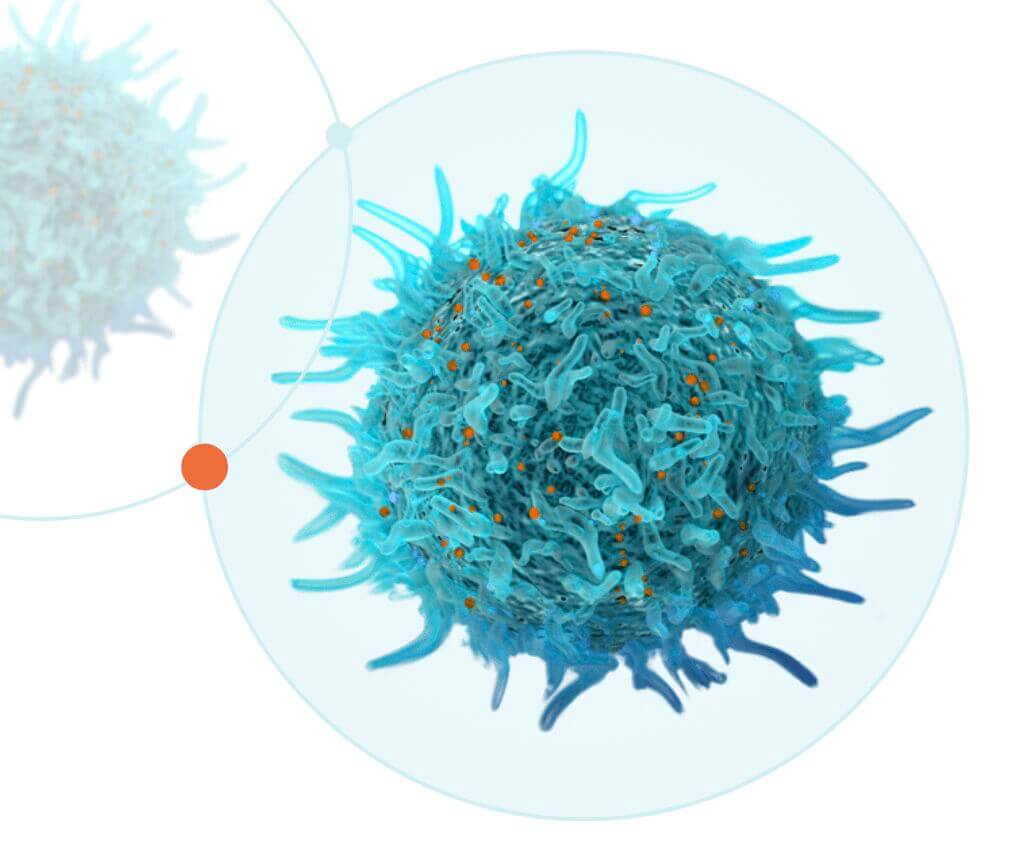
Moderna and Carisma establish collaboration to develop in vivo engineered Chimeric Antigen Receptor Monocytes (CAR-M) for Oncology
Moderna and Carisma establish collaboration to develop in vivo engineered Chimeric Antigen Receptor Monocytes (CAR-M) for Oncology
- Collaboration will combine Carisma’s engineered macrophage technology with Moderna’s mRNA and LNP technologies to generate and develop in vivo CAR-M therapeutics
- Multi-year research collaboration funded by Moderna with options for up to twelve targets
- Carisma to receive $45 million up-front cash payment and investment by Moderna in the form of a $35 million convertible note
- Carisma eligible to receive milestone and royalty payments
CAMBRIDGE, MA, and PHILADELPHIA, PA – January 10, 2022 – Moderna Inc. (NASDAQ:MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, and Carisma Therapeutics Inc., a biopharmaceutical pioneer in engineered macrophage-based therapeutics, today announced that the two companies have entered into a strategic collaboration agreement to discover, develop and commercialize in vivo engineered chimeric antigen receptor monocyte (CAR-M) therapeutics for the treatment of cancer.
“We are excited to begin this collaboration with Carisma to further expand our oncology pipeline with a differentiated in vivo cell-therapy approach,” said Stephen Hoge, President of Moderna. “This exemplifies our strategy to partner with companies with deep biological expertise while leveraging Moderna’s core mRNA and LNP capabilities to further expand the reach of Moderna’s technology.”
“Moderna’s deep expertise in mRNA and LNP technologies opens up a potentially game-changing opportunity for engineered macrophages,” said Steven Kelly, President and Chief Executive Officer of Carisma. “In vivo delivery directly to monocytes and macrophages enables an off-the-shelf therapeutic approach that uses the patients’ own cells to provide a truly personalized treatment. By combining Carisma’s expertise in engineered macrophage biology and Moderna’s pioneering in vivo mRNA delivery technologies, we are excited about the potential of this novel therapeutic approach for treating cancer. We are thrilled to be working with Moderna.”
About the Collaboration
Under the terms of the agreement, Carisma will receive a $45 million up-front cash payment and an investment by Moderna in the form of a $35 million convertible note. Carisma will receive research funding and is eligible to receive development, regulatory, and commercial milestone payments, plus royalties on net sales of any products that are commercialized under the agreement. Carisma will be responsible for the discovery and optimization of development candidates while Moderna will lead the clinical development and commercialization of therapeutics resulting from the agreement. Moderna has the option to nominate up to twelve targets for development and commercialization.
About Moderna
In 10 years since its inception, Moderna has transformed from a research-stage company advancing programs in the field of messenger RNA (mRNA), to an enterprise with a diverse clinical portfolio of vaccines and therapeutics across six modalities, a broad intellectual property portfolio in areas including mRNA and lipid nanoparticle formulation, and an integrated manufacturing plant that allows for both clinical and commercial production at scale and at unprecedented speed. Moderna maintains alliances with a broad range of domestic and overseas government and commercial collaborators, which has allowed for the pursuit of both groundbreaking science and rapid scaling of manufacturing. Most recently, Moderna’s capabilities have come together to allow the authorized use of one of the earliest and most-effective vaccines against the COVID-19 pandemic.
Moderna’s mRNA platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, and has allowed the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, cardiovascular diseases and auto-immune diseases. Moderna has been named a top biopharmaceutical employer by Science for the past seven years. To learn more, visit www.modernatx.com.
About Carisma Therapeutics
Carisma is a biopharmaceutical company dedicated to developing a differentiated and proprietary cell therapy platform focused on engineered macrophages, cells that play a crucial role in both the innate and adaptive immune response. The first applications of the platform, developed in collaboration with the University of Pennsylvania, are autologous chimeric antigen receptor (CAR)-macrophages for the treatment of solid tumors. Carisma is headquartered in Philadelphia, PA.
For more information, please visit www.carismatx.com
Moderna Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including regarding: Moderna’s collaboration with Carisma to discover, develop and commercialize CAR-M therapeutics for the treatment of cancer; the terms of that collaboration; and the potential for the collaboration to lead to personalized cancer treatments. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include those risks and uncertainties described under the heading “Risk Factors” in Moderna’s most recent Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (SEC) and in subsequent filings made by Moderna with the SEC, which are available on the SEC’s website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna’s current expectations and speak only as of the date hereof.
Moderna Contacts:
Media:
Colleen Hussey
Director, Corporate Communications
617-335-1374
Investors:
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
617-209-5834
Carisma Media Contact:
Creative Balloons appoints David Johnson as Chairman of the Board of Directors
Creative Balloons appoints David Johnson as Chairman of the Board of Directors
Creative Balloons GmbH, Waghäusel near Heidelberg, a specialist in medical technology, today announced the appointment of David Johnson as Chairman of the Board of Directors, effective January 1, 2022.
Creative Balloons appoints David Johnson as Chairman of the Board of Directors
|
DGAP-News: Creative Balloons GmbH / Key word(s): Personnel Creative Balloons appoints David Johnson as Chairman of the Board of Directors
Waghaeusel (Germany), January 10, 2022 – Creative Balloons GmbH, Waghäusel near Heidelberg, a specialist in medical technology, today announced the appointment of David Johnson as Chairman of the Board of Directors, effective January 1, 2022. Dave has held many senior executive and board positions with large market-leading companies as well as emerging companies with innovative medical devices. Managing global medtech businesses for over 20 years in Canada, the USA and the UK, he has successfully developed mid-, small- and micro-cap companies in public and private environments. “We are delighted to welcome David Johnson as Chairman of the Creative Balloons’ Board of Directors,” said Frank Gehres, CEO of Creative Balloons. “His many years of executive and board experience with large and smaller players in the medtech industry will be a tremendous asset for Creative Balloons as we approach market entry into the US and UK. Dave’s strategic advice and strong industry expertise, specifically in the intensive care segment, as well as his deep insight into the US market will help us to accomplish important tasks such as building up our commercial organization and achieving market access for our product hygh-tec(R). I look forward to working closely with him and our Board as we carry out our mission to rethink critical care.” David Johnson has 35 years of experience leading small, medium, and large companies – both public and private – in the medical device space, including intensive care products. Before heading the Creative Balloons board, Dave served as CEO for Enveric Biosciences, and continues as a member of their Board of Directors. “I strongly believe in the highly innovative approach to catheters that Creative Balloons has developed with hygh-tec(R),” commented David Johnson. “Its successful market entry in Germany is impressive. I am very much looking forward to working with Creative Balloons as it grows into new markets and to bringing in my business experience from that specific product category. It will be a pleasure to help leverage the full potential of the contribution hygh-tec(R) brings to ICU caregivers and the enhanced treatment opportunities it holds for patients.” “The Board of Creative Balloons is excited about Dave Johnson joining and leading our efforts,” says Dr. Ulrich Wandschneider, former CEO of hospital operator Asklepios and current member of the board of directors of BioNTech, who is representing investment company Salvia on the Creative Balloons Board of Directors. Besides him and David Johnson, the Board consists of Dr. Karl Nägler representing Wellington Partners and Matthias Guth representing MIG Capital AG. About Creative Balloons Contact: Media relations: 10.01.2022 Dissemination of a Corporate News, transmitted by DGAP – a service of EQS Group AG. The DGAP Distribution Services include Regulatory Announcements, Financial/Corporate News and Press Releases. |