MMI Raises $110 Million in Series C Financing
MMI Raises $110 Million in Series C Financing
Largest ever investment in microsurgery will further MMI’s mission to transform open surgery with robotic technology
JACKSONVILLE, Fla.–(BUSINESS WIRE)–MMI (Medical Microinstruments, Inc.), a robotics company dedicated to increasing treatment options and improving clinical outcomes for patients with complex conditions, today announced that it has raised $110 million in Series C financing. The round, led by Fidelity Management & Research Company, marks the largest ever investment in microsurgery innovation.
The funds will support commercialization of the Symani® Surgical System in high-growth markets and continued investment in studies that generate clinical evidence and enable indication expansion. Investments will also accelerate advanced technology capabilities and enable MMI to scale its operational capabilities globally.
MMI and its existing investors, all of whom contributed to the Series C financing, see considerable opportunity for rapid growth. The company projects the market for eligible robotic microsurgical procedures will grow from 3 million to 22 million annually by 2028, driven primarily by technological advancements and indication expansion.
“Against a backdrop of plateauing investments in medical robotics, this support builds on our confidence in a new, less invasive solution for open surgery, a significant market that can benefit from the smallest wristed microinstruments,” said Mark Toland, CEO of MMI. “Our Symani Surgical System is uniquely positioned to expand patient access to care by accelerating the number of surgeons able to perform complex, delicate procedures. With the support of our investors, we will continue to advance our technology through a growing body of clinical evidence and expanded hospital partnerships.”
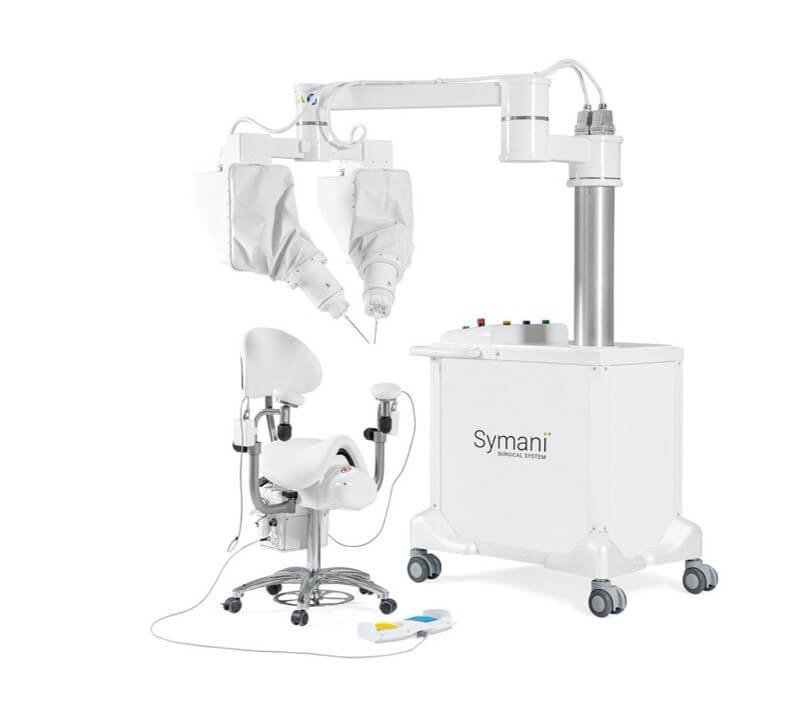
The Symani Surgical System is a first-of-its-kind robotic technology that uniquely addresses the scale and complexities of microsurgery and supermicrosurgery. By allowing surgeons to replicate the natural movements of the human hand at the micro scale, it can expand treatment options for patients in need of soft tissue, open surgical procedures, such as free flap reconstructions, lymphatic surgery, and trauma replantations. It is designed to help restore quality of life for more patients, accelerate the number of surgeons able to push the boundaries of complex procedures for delicate anatomy, and enable hospitals to expand their open surgical programs.
MMI has raised over $200 million in funding to date. In 2022, it closed a Series B financing round to propel growth. The funding was allocated to help expand indications and support ongoing commercialization efforts for the Symani Surgical System in Europe, where it received CE mark in 2019, accelerate plans to commercialize in the U.S. and Asia-Pacific, and advance clinical research.
To learn more about MMI and the Symani Surgical System, visit MMI’s website here: https://mmimicro.com.
About MMI
MMI (Medical Microinstruments, Inc.) is on a mission to advance robotic technology that pushes the limits of soft tissue open surgery and opens new opportunities for surgeons to restore quality of life for more patients with complex conditions. The company was founded in 2015 near Pisa, Italy, and its proprietary Symani® Surgical System combines the world’s smallest wristed microinstruments with tremor-reducing and motion-scaling technologies to address significant unmet patient needs across the globe. This first-of-its-kind surgical robotic platform for open, soft tissue micro-level surgery can help address microvascular repair, lymphatic repair, and peripheral nerve repair. The Symani System is CE Marked for commercial use in Europe. In the United States, the system is not approved or cleared for commercial use. MMI is backed by global investors including Fidelity Management & Research Company, Andera Partners, BioStar, Deerfield Management, Fountain Healthcare Partners, Panakès Partners, RA Capital, Sambatech, and Wellington Partners.

UroMems Announces Results of First-Ever Smart Artificial Urinary Sphincter Implant in Female Patient to Treat Stress Urinary Incontinence
UroMems Announces Results of First-Ever Smart Artificial Urinary Sphincter Implant in Female Patient to Treat Stress Urinary Incontinence
Positive results demonstrate promising new option for women seeking better, personalized treatment
GRENOBLE, France and MINNEAPOLIS, Feb. 14, 2024 /PRNewswire/ — UroMems, a global company developing innovative, mechatronics technology to treat stress urinary incontinence (SUI), announced today that it has successfully met the six-month primary endpoint for the first-ever female patient implanted with the UroActive™ System, the first smart automated artificial urinary sphincter (AUS) to treat SUI. This milestone indicates a new era for millions of women suffering from SUI, and signals an exciting transition for surgeons treating SUI not only in France, where the female patient was treated, but also across Europe and the U.S. It also follows closely on the heels of the successful results of the complete treatment cohort of the first-in-man clinical feasibility study. Results of this clinical study will contribute to the design and implementation of UroMems’ pivotal clinical trial in Europe and the U.S.
“On behalf of the medical team who implanted the UroActive System in the first female patient, Drs. Christophe Vaessen, Aurelien Beaugerie and I are over the moon to see our first patient has returned to living life fully after years of struggling with SUI,” said Professor Emmanuel Chartier-Kastler. “This promising therapy is a breakthrough technology in treating SUI in both women and men.”
The primary outcome measures include the successful device activation and the rate of explants and revisions at six months. The first female patient has not only met the study’s primary endpoints by remaining revision-free but is also experiencing restored social continence. Follow-up on secondary measures, including leak rate values, has been extremely positive.
“As the leading advocacy organization in the U.S. supporting patients and caregivers with urinary incontinence, we hear from patients weekly looking for information on the safety and efficacy of artificial sphincters. Unfortunately, we have little to report back and support their requests as no one in the U.S. actively promotes this option for women,” said Steven Gregg, Ph.D., executive director of the National Association for Continence. “These results of the first UroActive System implanted in a female represent a promising development to treat stress urinary incontinence in both women and men. We’re excited about this first-of-its-kind development and look forward to UroMems’ pivotal trial results.”
Pending those results, the potential for U.S. surgeons to offer this new option is now on the horizon; skilled surgeons performing robotic-assisted surgeries such as sacrocolpopexies and hysterectomies may soon be able to add implanting UroActive to their standard practices. UroActive is the first smart active implant that treats SUI, powered by a MyoElectroMechanical System (MEMS). This innovative system is placed around the urethra in men and the bladder neck in women, controlled based on the patient’s activity, without the need for manual adjustments, intending to provide patients with ease of use and a better quality of life than current options.
“We are elated to reach this critical achievement contributing to the demonstration of the feasibility of the UroActive System to successfully treat women suffering from debilitating SUI,” said Hamid Lamraoui, UroMems chief executive officer and co-founder. “The compelling results of this first-in-female implant show the high potential of our technology, bringing us one step closer to delivering on the massive unmet need for women and physicians desperately seeking a better SUI treatment option.”
SUI, or involuntary urinary leakage, affects an estimated 40 million Americans and 90 million Europeans, and occurs when the pressure in the bladder exceeds that of the muscle (the sphincter) around the urethra, caused by activities involving high intra-abdominal pressure, like coughing, laughing and exercising. SUI significantly impacts quality of life, as it can be debilitating, and often leads to depression, low self-esteem and social stigma.
UroMems aims to restore the quality of life, dignity and self-esteem of millions of men and women worldwide suffering from poorly treated chronic conditions by the commitment to change the perception that these disorders are inevitable as one grows older and is simply something to endure with no real solution. UroMems is revolutionizing the treatment of SUI with smart active implants, using the latest technological advances in the field of embedded systems and micro-technologies for the development of its groundbreaking solutions.
About UroActive
UroActive is an active implantable electronic artificial urinary sphincter that is being developed to compensate for sphincter insufficiency in patients, both men and women, with SUI. It is based on a unique bionic platform using embedded smart, digital and robotic systems which, based on data collected from a patient, create a treatment algorithm that is specific for each patient’s needs. The UroMems technology platform is protected by more than 120 patents and is designed to overcome the limitations of current solutions by optimizing safety and performance, patient experience and surgeon convenience. STeP participation does not imply product authorization. UroActive has not received marketing authorization from the FDA and is not available for sale in the United States or in the EU.
For more information, please visit www.uromems.com.
Media Contact:
Shelli Lissick
651-276-6922