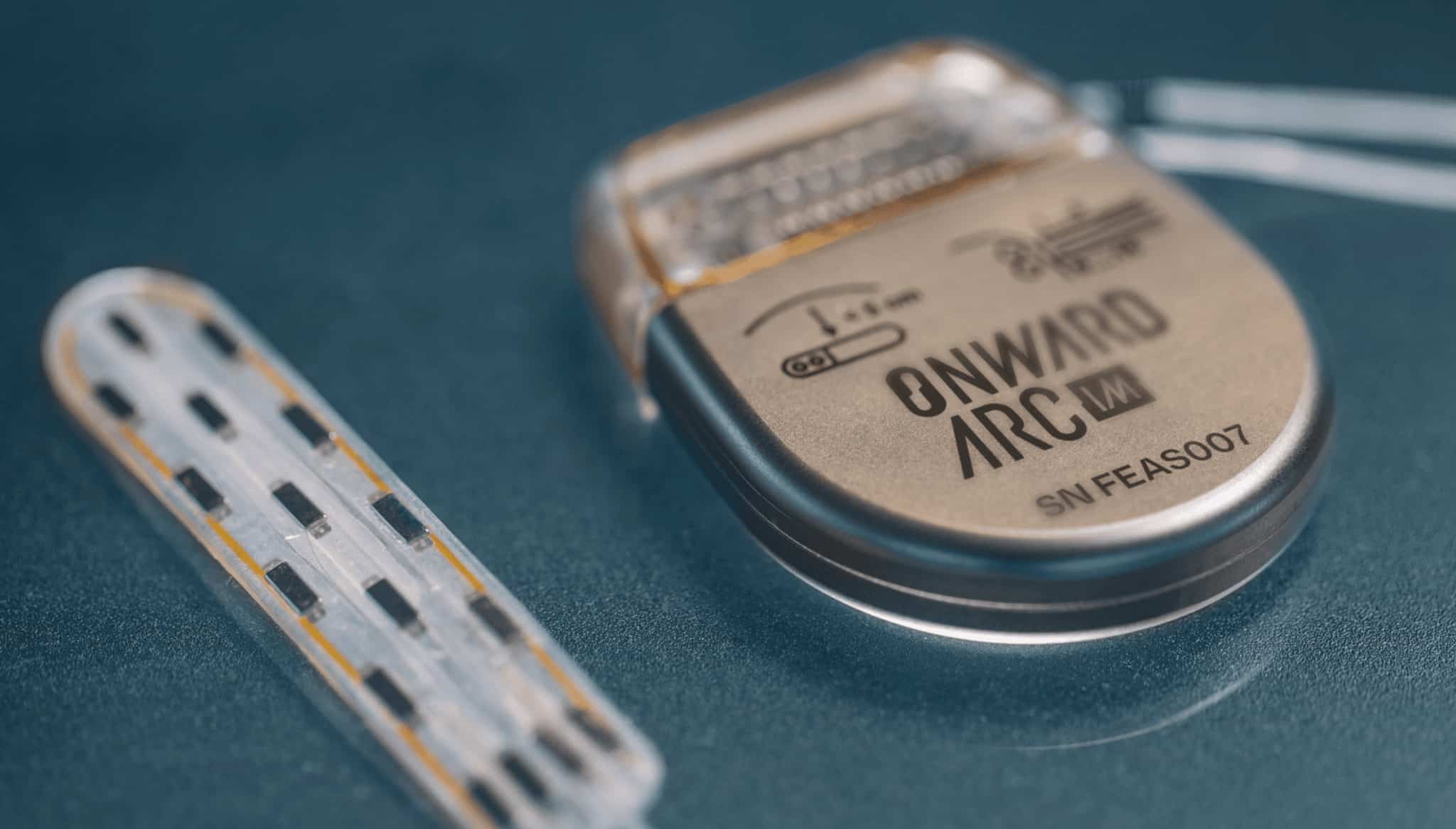
ONWARD adds new brain-spine interface and Parkinson’s disease IP to license agreements
ONWARD Adds New Brain-Spine Interface and Parkinson’s Disease IP to License Agreements
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—March 28, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury (SCI), today announces two new innovations have been added to its portfolio of license agreements with the Swiss Federal Institute of Technology Lausanne (EPFL), one of the world’s preeminent neuroscience research institutions, and Lausanne University Hospital (CHUV), ranked among the top 10 hospitals in the world.
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—March 28, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury (SCI), today announces two new innovations have been added to its portfolio of license agreements with the Swiss Federal Institute of Technology Lausanne (EPFL), one of the world’s preeminent neuroscience research institutions, and Lausanne University Hospital (CHUV), ranked among the top 10 hospitals in the world.
Adrenomed Supervisory Board appoints Dr. Richard Jones as CEO
Adrenomed Supervisory Board appoints Dr. Richard Jones as CEO
Adrenomed Supervisory Board appoints Dr. Richard Jones as CEO
Hennigsdorf/Berlin (Germany), 15 March, 2022 – Adrenomed AG, the vascular integrity company, today announced the appointment of Dr. Richard Jones as Chief Executive Officer of Adrenomed, effective today. Dr. Jones brings over 25 years of experience in leadership positions in the pharmaceutical and biotech industry with a sustained record of achievements in business, clinical development and commercialization.
“I am delighted to welcome Dr. Richard Jones as the new CEO of Adrenomed. He is a highly achieved life science specialist, who provides a unique combination of strategic, financial, development and organizational capabilities to the Company. Under his leadership, Adrenomed has a strong team in place to lead its first-in-class drug candidate Adrecizumab through late-stage clinical development towards commercialization. Our goal is to bring an urgently needed, effective precision medicine for treating sepsis to intensive care units,” said Prof. Dr. Erich Schlick, Chairman of Adrenomed’s Supervisory Board.
“On behalf of the entire Supervisory Board, I would also like to thank Dr. Wolfgang Baiker, former CEO of Adrenomed, for his contribution to the company and wish him well for the future,” he added.
Dr. Richard Jones, CEO of Adrenomed, said: “I am excited to be joining Adrenomed at a pivotal phase of the Company’s evolution. Sepsis is responsible for between one third and one half of deaths of hospitalized people. This equates to 11 million people worldwide dying each year which is higher than the top 3 cancer conditions combined and represents a significant high unmet medical need. Adrenomed’s innovative biomarker guided treatment approach, with Adrecizumab, is targeting the endothelial barrier dysfunction as a major cause of mortality in sepsis. I look forward to working with the team and advancing the late-stage clinical development of Adrecizumab to provide a new treatment option for critically ill patients.”
Richard Jones joins Adrenomed from Fusion Antibodies Plc, a contract research organization specialized in antibody engineering. As CEO of Fusion Antibodies Plc, Richard successfully implemented a new growth strategy in the global antibodies marketplace. Previously, Richard served as CEO for a number of UK and European private companies as well as SVP, Head of Europe for a US based public company. Before this he gained expertise at Novartis and GSK as VP, Medicines Commercialization Leader Global Haematology at both companies. In these roles, Richard was responsible for three regulatory submissions and initiation of several new drug development programs, plus the planning and execution of three global product launches. Richard also worked with Genzyme Corporation and as International Franchise Director for Shire Pharmaceuticals. He holds a BSc in Biochemistry and PhD in Molecular Oncology from University of Surrey.
About Adrenomed
Adrenomed AG is a German privately financed, clinical-stage biopharmaceutical company. Adrenomed’s mission is to rescue vascular integrity in order to save the lives of critically ill patients with limited treatment options. Founded in 2009 by a management team with decades of in-depth experience in sepsis and deep knowledge in diagnostics and drug development, the company’s lead product candidate Adrecizumab is a first-in-class monoclonal antibody. Adrecizumab targets the vasoprotective peptide Adrenomedullin, an essential regulator of vascular integrity. Adrecizumab has successfully completed a biomarker-guided, double-blinded, placebo-controlled, randomized, multicenter proof-of-concept Phase II trial with 301 patients suffering from septic shock. For further information, please visit www.adrenomed.com and follow us on LinkedIn and Twitter.
Contact
Adrenomed AG
Frauke Hein, Ph.D. (Chief Business Officer)
phone: +49 (0)3302 2077814
Media Inquiries
MC Services AG
Eva Bauer / Julia von Hummel
phone: +49 (0)89 21022880
First participants enrolled in ONWARD’s LIFT home study
First participants enrolled in ONWARD’s LIFT home study
Study will evaluate safety and performance of ARCEX therapy for spinal cord injury in home setting
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—March 9, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury, today announces the start of enrollment in the LIFT Home Study. The trial is designed to study the safety and performance of ARCEX Therapy when used in the home.
ONWARD’s ARCEX Therapy is externally delivered programmed stimulation of the spinal cord to restore strength and function in people with spinal cord injury and other movement related challenges. The study will enroll up to 20 participants at 5 leading research centers in the United States: Craig Hospital in Denver, Colorado, Shepherd Center in Atlanta, Georgia, Spaulding Research Institute in Boston, Massachusetts, University of Minnesota, and University of Washington. Subjects will be followed for four weeks to assess whether continued access to ONWARD’s ARCEX Therapy is safe and can be used to enhance long-term benefit.
The first participants have been enrolled at Craig Hospital under the supervision of study principal investigator, Candy Tefertiller, PT, DPT, Ph.D., NCS, Executive Director of Research and Evaluation at Craig and at the University of Washington in Seattle by Chet Moritz, PhD, Associate Professor in the Departments of Electrical& Computer Engineering and Rehabilitation Medicine. “The LIFT Home Study is an important next step in understanding the potential benefits people with spinal cord injury may derive from continued access to ARC Therapy outside the clinic,” said Dr. Tefertiller.
The LIFT Home Study is a successor to ONWARD’s Up-LIFT Study, a pivotal trial that completed enrollment of 65 participants in December 2021, with participating research centers in the US, Canada, the UK, and Europe. Up-LIFT is designed to demonstrate ONWARD ARCEX Therapy can improve the strength and function of upper limbs when used in the rehabilitation clinic setting.
The LIFT Home Study seeks to evaluate the potential impact of ARC EX Therapy when used in the home setting.
“We are pleased to collaborate with an outstanding group of researchers to investigate new potential benefits and care settings for our ARC EX Therapy,” said Dave Marver, CEO of ONWARD. “This is another step in our journey to help people with spinal cord injury live better, more independent lives.”
To learn more about ONWARD’s ARC Therapy and the company’s vision to restore movement, independence and health in people with spinal cord injury, please visit ONWD.com.
About ONWARD
ONWARD is a medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury. ONWARD’s work builds on more than a decade of basic science and preclinical research conducted at the world’s leading neuroscience laboratories. ONWARD’s ARC Therapy, which can be delivered by implantable (ARC IM) or external (ARCEX ) systems, is designed to deliver targeted, programmed stimulation of the spinal cord to restore movement and other functions in people with spinal cord injury, ultimately improving their quality of life. ONWARD has received three Breakthrough Device Designations from the FDA encompassing both ARCIM and ARCEX . The company’s first FDA pivotal trial, called Up-LIFT, completed enrollment in December 2021 with 65 subjects worldwide.
ONWARD is headquartered at the High Tech Campus in Eindhoven, the Netherlands. It maintains a significant team in Lausanne, Switzerland and has a growing U.S. presence in Boston, Massachusetts, USA. For additional information about the company, please visit ONWD.com. To access our 2022 Financial Calendar, please visit IR.ONWD.com.
For Media Enquiries:
Simon Gentry
+44 (0)20 3757 6772
For Company Enquiries:
ONWARD
For Investor Enquiries:
Disclaimer
Certain statements, beliefs and opinions in this press release are forward-looking, which reflect the Company or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve a number of risks, uncertainties and assumptions that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, changes in demand, competition and technology, can cause actual events, performance or results to differ significantly from any anticipated development. Forward looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.