NEUWAY Pharma and WACKER Launch Research Project to Develop RNA-Based Actives for the Treatment of Central Nervous System Diseases
Joint Press Release by WACKER and NEUWAY Pharma
Munich and Bonn, Oct 12, 2022- Bonn-based biotech company NEUWAY Pharma and Munich-based chemical company WACKER have launched a research project to identify and manufacture RNA-based actives for the treatment of diseases of the central nervous system (CNS). They will make use of NEUWAY’s protein-based drug delivery technology EnPC®. EnPC® allows for targeted drug delivery via the blood-brain barrier into the CNS.
Under the collaboration agreement, NEUWAY Pharma and WACKER intend to jointly research active substances based on ribonucleic acid (RNA) for the treatment of CNS-related diseases; the companies will also study related manufacturing processes. They will make use of NEUWAY Pharma’s protein-based drug delivery technology EnPC® (Engineered Protein Capsules), where active ingredient molecules are encapsulated by protein capsules and thereby transported across the blood-brain barrier – which is difficult to pass – into the CNS.
The blood-brain barrier serves as natural protection against harmful substances. Crossing it has so far posed a major challenge for the treatment of CNS diseases. EnPC® now makes effective transport of drugs into the CNS possible. This technology also offers a range of advantages for RNA-related therapies. Alongside lower costs in manufacturing, dosing of drugs based on EnPC® can be handled flexibly, for instance. Intravenous administration is furthermore possible, which eases the burden on clinicians, healthcare providers, payors and patients.
Within the research project, WACKER will take on the production and analysis of various mRNA grades, in particular mRNA – mRNA (messenger RNA) is a special type of RNA made from DNA. NEUWAY is responsible for manufacturing the EnPCs, the encapsulation and the design of selected RNA compounds, as well as associated (bio-)analysis and investigation of therapeutic relevance, both in vitro and in vivo.
“We see great opportunities in the joint research project with WACKER. WACKER’s expertise in the production of mRNA supports our strategy of advancing transformative neuropharmaceuticals for the treatment of diseases of the central nervous system,” says Oliver Ernst, CEO and managing director of NEUWAY Pharma. “We are pleased that, with our expertise, we can contribute to research into new treatment methods for diseases of the central nervous system. The combination of mRNA-based drugs with NEUWAY’s delivery system has great therapeutic potential,” says Hagen Richter, who is responsible for research in the area of nucleic acids at WACKER. The molecular biologist is in charge of the project on WACKER’s side.
About NEUWAY Pharma
NEUWAY Pharma is a German biotech company based in Bonn. It is developing an entirely novel class of biotherapeutics, based on its patent-protected Engineered Protein Capsules (EnPC®). These can cross the blood-brain barrier and deliver innovative neuropharmaceuticals for the treatment of diseases of the central nervous system (CNS). Find out more at www.neuway-pharma.com.
About WACKER
Wacker Chemie AG is a global company with state-of-the-art specialty chemical products found in countless everyday items, ranging from cosmetic powders to solar cells. WACKER’s portfolio comprises more than 3,200 products supplied in over 100 countries. WACKER has a global network of 27 production sites, 23 technical competence centers and 52 sales offices. In 2021, the Group’s 14,400 employees generated global sales of €6.21 billion. Wacker Chemie AG is listed on the Deutsche Boerse Prime Standard and on the MDAX (ISIN: DE000WCH8881). Find out more at www.wacker.com.
Contact
Wacker Chemie AG
Manuela Dollinger
Tel.: +49 89 6279-1629
NEUWAY Pharma
Christine Kuhn
Tel.: +49 228-522198-0
Immatics Reports Interim Clinical Data Update on ACTengine® IMA203 TCR-T Monotherapy Targeting PRAME
Immatics Reports Interim Clinical Data Update on ACTengine® IMA203 TCR-T Monotherapy Targeting PRAME
Immatics Reports Interim Clinical Data Update on ACTengine® IMA203 TCR-T Monotherapy Targeting PRAME
- Clinical validation of PRAME as multi-tumor target with large potential for TCR-based therapies: confirmed responses in different solid cancers, in patients with high and low PRAME expression
- Update covers data from 27 patients in completed Phase 1a dose escalation and first 5 patients in Phase 1b dose expansion (cohort A) treated with IMA203 monotherapy
- Confirmed objective response rate (cORR): 50% (6/12) at target dose or above with at least 1 billion infused TCR-T cells across Phase 1a and 1b; thereof 80% cORR (4/5) in Phase 1b patients alone with all responses ongoing at data cut-off
- Confirmed responses across different solid tumor types: cutaneous melanoma, ovarian cancer, head and neck cancer, uveal melanoma, and synovial sarcoma
- Treatment with IMA203 continues to show manageable tolerability; biological data including T cell engraftment, persistence and tumor infiltration consistent with clinical data
- IMA203 TCR-T is part of Immatics’ strategy to leverage the full clinical potential of targeting PRAME; next data read-outs on IMA203 monotherapy, IMA203 in combination with a checkpoint inhibitor and 2nd generation IMA203CD8 planned during 2023
Houston, Texas and Tuebingen, Germany, October 10, 2022 – Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, today announced a clinical data update for the IMA203 monotherapy covering the completed Phase 1a dose escalation part of the trial and initial data from the first 5 patients in the ongoing Phase 1b dose expansion cohort A (monotherapy). In the Phase 1 trial with ACTengine® IMA203, Immatics is treating recurrent and/or refractory solid cancer patients utilizing TCR-T cells directed against an HLA-A*02-presented peptide derived from PRAME, which is frequently expressed across several solid cancer indications. Overall, IMA203 continues to be well tolerated and achieved confirmed objective responses across multiple solid cancers such as cutaneous melanoma, ovarian cancer, head and neck cancer, uveal melanoma, and synovial sarcoma. Encouraging early signs of improved durability were seen with a 50% (6/12) confirmed objective response rate, when patients were infused at the target dose or above with more than 1 billion TCR-T cells.
Key clinical findings from IMA203 TCR-T monotherapy
The data obtained during the Phase 1a and Phase 1b cohort A trial provide clinical validation of PRAME as a highly promising T cell target for solid cancers. Confirmed clinical responses were observed at high and low PRAME-expression levels above threshold, indicating IMA203’s potential to provide clinical benefit for all PRAME biomarker-positive cancer patients. The predicted high PRAME prevalence across key indications has so far been supported by prevalence rates obtained during the clinical screening of patients.
Moving from Phase 1a to Phase 1b, Immatics has continued to introduce planned improvements that may influence clinical outcomes including (1) applying higher cell doses (DL4 and exploratory DL5), (2) optimizing the cell product through manufacturing enhancements and (3) working with disease area experts to gradually reduce the fraction of very heavily pre-treated patients with extreme tumor burden who have exhausted standard of care and have undergone multiple clinical trials. In addition, the focus in Phase 1b is also shifting from initial objective response rate (ORR) determined at the ~6-week scan to confirmed ORR determined at the ~12-week scan.
Preliminary Objective Response Rates (ORR; RECIST 1.1) in Phase 1a and Phase 1b Cohort A
| Phase 1a | Phase 1a + Phase 1b | Phase 1b only | ||
| All pts (DL1-4) | DL4 pts only 1 | DL4/DL5 pts only1 | All pts (DL4/DL5)1 | |
| Patients Treated | 27 | 7 | 12 | 5 |
| ORR (~week 6) | 48% (13/27) | 57% (4/7) | 67% (8/12) | 80% (4/5) |
| cORR (~week 12)2 | 19% (5/27) | 29% (2/7) | 50% (6/12)* | 80% (4/5)* |
1 All patients received >1 billion total TCR-T cells; 2confirmed ORR (cORR), * 1 patient with SD at ~6-week scan with pending ~12-week scan considered as non-responder for cORR; DL – dose level
Positively evolving durability profile for IMA203 was observed at higher doses: 6 of 12 patients (50%) treated with more than 1 billion infused TCR-T cells (DL4 and DL5) in the Phase 1a and Phase 1b cohort A part of the trial experienced a confirmed objective response (partial response according to RECIST 1.1). In the Phase 1b part of the trial alone, 4 of 5 patients (80%) had a confirmed objective response which were all ongoing at the timepoint of data cut-off.
“The data presented today highlight the clinical potential of PRAME as one of the most promising multi-tumor targets to achieve meaningful benefits for a large cancer patient population,” commented Cedrik Britten, MD, Chief Medical Officer at Immatics. “In addition to this first data from IMA203 monotherapy today, we are awaiting data from two additional dose expansion cohorts: IMA203 together with an immune checkpoint inhibitor and our 2nd generation product candidate IMA203CD8. As we continue to shift our focus from Phase 1a to Phase 1b, we look forward to reporting meaningful data throughout 2023, including safety and response rates, as well as durability of response with a longer follow-up time. In addition, we are excited to start a first-in-human trial with our half-life extended Bispecific against PRAME, TCER® IMA402, also in 2023.”
Safety data for IMA203 monotherapy across Phase 1a and Phase 1b: Treatment with IMA203 continues to show manageable tolerability profile.
- At data cut-off on September 6, 2022, 32 patients were infused with IMA203 TCR-T cells.
- Most frequent treatment-emergent adverse events (TEAEs) were as expected for cell therapies.
- All patients experienced expected cytopenia (Grade 1-4) associated with lymphodepletion. 31 patients (97%) experienced cytokine release syndrome (CRS) of any grade: 29 patients had low to moderate (Grade 1-2), and 2 patients had Grade 3 CRS that occurred in Phase 1a; both recovered to Grade ≤2 after 3 and 4 days. 5 patients (16%) experienced a low to moderate (Grade 1-2) immune effector cell associated neurotoxicity syndrome (ICANS). No dose-dependent increase of CRS and ICANS was observed.
- No additional dose limiting toxicities (DLT) were observed since the initial data release in March 2021.
Phase 1a – Clinical activity: IMA203 demonstrated a high initial objective response rate in several solid tumor types.
- At data cut-off on September 6, 2022, a total of 27 patients received IMA203 monotherapy in the Phase 1a dose escalation trial:
- High initial objective response rate (ORR; partial responses according to RECIST 1.1) of 48% (13/27) was observed at the first CT scan post infusion at ~week 6, and a confirmed ORR of 19% (5/27) the second CT scan at ~week 12.
- 7 out of 27 patients received doses above 1 billion TCR-T cells (DL4); initial ORR was 57% (4/7) and confirmed ORR was 29% (2/7) in these patients.
- Patients were heavily pre-treated with a mean of 4.2 lines of prior systemic treatment and a particularly high baseline tumor burden.
- The provisional recommended Phase 2 dose (RP2D) for Phase 1b dose expansion was determined to be DL4.
Phase 1b Cohort A – Clinical activity: IMA203 monotherapy demonstrates high confirmed objective response rate of 80% with early signs of prolonged durability.
- At data cut-off on September 6, 2022, 5 patients received IMA203 monotherapy at DL4 and DL5 in the Phase 1b cohort A dose expansion trial:
- 4 out of 5 patients (80%) experienced an initial objective response at ~week 6 (PR according to RECIST 1.1).
- In all 4 patients, objective responses were confirmed at ~week 12 and were ongoing at data cut-off: confirmed ORR was 80% (4/5).
- All 4 responses were observed in different solid tumor types: cutaneous melanoma, ovarian cancer, uveal melanoma and head and neck cancer.
- Patients were heavily pre-treated with a mean of 4.0 lines of prior systemic treatment and high to moderate baseline tumor burden.
ACTengine® IMA203 is currently being evaluated in an ongoing Phase 1b study including three expansion cohorts: (A) IMA203 as a monotherapy, (B) IMA203 in combination with an immune checkpoint inhibitor and (C) IMA203CD8, a next-generation cell therapy where IMA203 engineered T cells are co-transduced with a CD8αβ co-receptor. Further data read-outs on the individual cohorts are planned throughout 2023. In addition to the ACTengine® programs, Immatics is addressing PRAME-positive cancers with a second therapeutic modality: TCR Bispecifics. The company’s TCER® IMA402 is a next-generation, half-life extended TCR Bispecific which will enter the clinic in 2023. Both approaches, ACTengine® and TCER®, are distinct therapeutic modalities that have the potential to provide innovative treatment options for a variety of cancer patient populations with different medical needs.
Immatics conference call
Immatics will host a conference call today, October 10, 2022, at 8:30 am EDT / 2:30 pm CEST to discuss these clinical data. The webcast and presentation can be accessed directly through this link. Participants may also access the slides and the webcast on the Immatics website in the Investors section under “Presentations” at www.investors.immatics.com/events-presentations. A replay of the webcast will be made available shortly after the conclusion of the call and archived on the Company’s website for at least 90 days.
About IMA203 and target PRAME
ACTengine® IMA203 T cells are directed against an HLA-A*02-presented peptide derived from preferentially expressed antigen in melanoma (PRAME), a protein frequently expressed in a large variety of solid cancers, thereby supporting the programs’ potential to address a broad cancer patient population. Immatics’ PRAME peptide is present at a high copy number per tumor cell and is homogenously and specifically expressed in tumor tissue. The peptide has been identified and characterized by Immatics’ proprietary mass spectrometry-based target discovery platform XPRESIDENT®. Through its proprietary TCR discovery and engineering platform XCEPTOR®, Immatics has generated a highly specific T cell receptor (TCR) against this target for its TCR-based cell therapy approach, ACTengine® IMA203.
About ACTengine®
ACTengine® is a personalized cell therapy approach for patients with advanced solid tumors. The patient’s own T cells are genetically engineered to express a novel, proprietary TCR directed against a defined cancer target. The modified T cells are then reinfused into the patient to attack the tumor. The approach is also known as TCR-engineered cell therapy (TCR-T). All Immatics’ ACTengine® product candidates can be rapidly manufactured utilizing a proprietary manufacturing process designed to enhance T cell engraftment and persistence in vivo.
The ACTengine® T cell products are manufactured at the Evelyn H. Griffin Stem Cell Therapeutics Research Laboratory in collaboration with UTHealth. The ACTengine® Programs are co-funded by the Cancer Prevention and Research Institute of Texas (CPRIT).
– END –
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
For regular updates about Immatics, visit www.immatics.com. You can also follow us on Twitter and LinkedIn.
Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the timing of product candidates and Immatics’ focus on partnerships to advance its strategy are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
| Media and Investor Relations Contact | |
| Jacob Verghese or Eva Mulder | |
| Trophic Communications | |
| Phone: +49 89 2070 89831 or +31 65 2331 579 | |
| Immatics N.V. | |
| Anja Heuer | Jordan Silverstein |
| Director, Corporate Communications | Head of Strategy |
| Phone: +49 89 540415-606 | Phone: +1 281 810 7545 |
Immatics Announces $110 Million Underwritten Offering of Ordinary Shares
Immatics Announces $110 Million Underwritten Offering of Ordinary Shares
Immatics Announces $110 Million Underwritten Offering of Ordinary Shares
Houston, Texas and Tuebingen, Germany, October 10, 2022 – Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, announced today that it has agreed to sell, by way of an underwritten public offering, 10,905,000 of its ordinary shares at a price of $10.09 per share. The gross proceeds from the offering, before deducting the underwriting discount and offering expenses, are expected to be approximately $110 million. The offering is expected to close on October 12, 2022, subject to customary closing conditions.
The offering included participation from investors including Armistice Capital Master Fund Ltd., Dellora Investments, EcoR1 Capital, Nantahala Capital, Perceptive Advisors, Rock Springs Capital, RTW Investments, LP, Samsara BioCapital, SilverArc Capital, Sofinnova Investments, Wellington Management, 683 Capital and other specialist biotech investors.
Jefferies and SVB Securities are acting as joint book-running managers for the offering.
A registration statement relating to these securities has been filed with the U.S. Securities and Exchange Commission (the “SEC”) and was declared effective on August 9, 2021. The offering is being made only by means of a prospectus supplement and accompanying prospectus. When available, copies of the final prospectus supplement and accompanying prospectus related to the offering may be obtained for free from Jefferies LLC, Attention: Equity Syndicate Prospectus Department, 520 Madison Avenue, 2nd Floor, New York, NY 10022, telephone: (877) 821-7388, email: ; or SVB Securities LLC, Attention: Syndicate Department, 53 State Street, 40th Floor, Boston, MA 02109, telephone: (800) 808-7525, ext. 6105, email: .
This press release shall not constitute an offer to sell or a solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Any offers, solicitations or offers to buy, or any sales of securities will be made in accordance with the registration requirements of the Securities Act of 1933, as amended.
– END –
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the closing of the public offering and gross proceeds therefrom are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements.
For more information, please contact:
| Media and Investor Relations Contact | |
| Jacob Verghese or Eva Mulder | |
| Trophic Communications | |
| Phone: +49 89 2070 89831 or +31 65 2331 579 | |
| Immatics N.V. | |
| Anja Heuer | Jordan Silverstein |
| Director, Corporate Communications | Head of Strategy |
| Phone: +49 89 540415-606 | Phone: +1 281 810 7545 |
ONWARD Announces Appointment of Vivian Riefberg to Board of Directors
ONWARD Announces Appointment of Vivian Riefberg to Board of Directors
EINDHOVEN, the Netherlands, LAUSANNE, Switzerland, and BOSTON, MA USA—September 26, 2022—ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury, today announced the appointment of Vivian Riefberg as a non-executive member of its Board of Directors. Ms. Riefberg will join the Board immediately and will serve on its Compensation Committee. Her appointment to the Board will be presented for shareholder approval at the next Annual General Meeting.
“Vivian Riefberg is a distinguished expert in health care, government, and strategy. I am excited and honored to welcome her to our Board”, said Dave Marver, Chief Executive Officer. “Vivian brings a rich understanding of the institutions with which we will collaborate to bring our novel therapies to market, including the U.S. Veterans Health Administration. Her experience and insights will be invaluable as we work to create an entirely new therapeutic domain in neuromodulation and help people with spinal cord injury and other movement disabilities.”
“ONWARD has the opportunity to build a very special enterprise that is both successful and impactful”, said Vivian Riefberg. “I have had the privilege to counsel many leading companies during my career. ONWARD has a chance to help people with spinal cord injury by leveraging groundbreaking science, technology, and intellectual property, fueled by a fullhearted mission.”
In 2020, Ms. Riefberg retired as a senior partner with McKinsey & Company, where she held a variety of senior positions, including leader of the Public Sector Practice for the Americas and co- leader of the U.S. Health Care practice. She served on McKinsey & Company’s global board of directors and on the committee evaluating and developing global partners. Ms. Riefberg is currently Professor of Practice at the University of Virginia Darden School of Business where she holds the David C. Walentas Jefferson Scholars Chair. At Darden, Ms. Riefberg focuses on healthcare, consulting, and strategy across the private, public and not-for-profit sectors. Her emphasis is senior executive leadership, including leading during times of uncertainty and crisis, solutions and innovations in healthcare, and women’s leadership.
Ms. Riefberg serves on the Board of Signify Health, K Health and Lightrock, an impact investing firm, as well as of the Public Broadcasting System (PBS), Johns Hopkins Medicine, the Lorna Breen Heroes Foundation and the National Education Equity Lab. She is also an advisory board member for the Smithsonian’s planned American Women’s History Museum. She previously served on the U.S. National Institutes of Health (NIH) Clinical Center Board of Governors and on the NIH Advisory Board for Clinical Research. She also served on the Board of Directors of the Partnership for a Healthier America (PHA), a nonprofit, created to mobilize efforts to solve the child obesity challenge as an outgrowth of First Lady Michelle Obama’s Let’s Move campaign.
Ms. Riefberg is a frequent speaker at various conferences including the World Economic Forum at Davos. She is also a contributor to leading industry publications on improving U.S. healthcare, addressing government productivity and women’s leadership.
She holds a B.A., magna cum laude in history from Harvard-Radcliffe College and an M.B.A. with distinction from Harvard Business School.
About ONWARD Medical
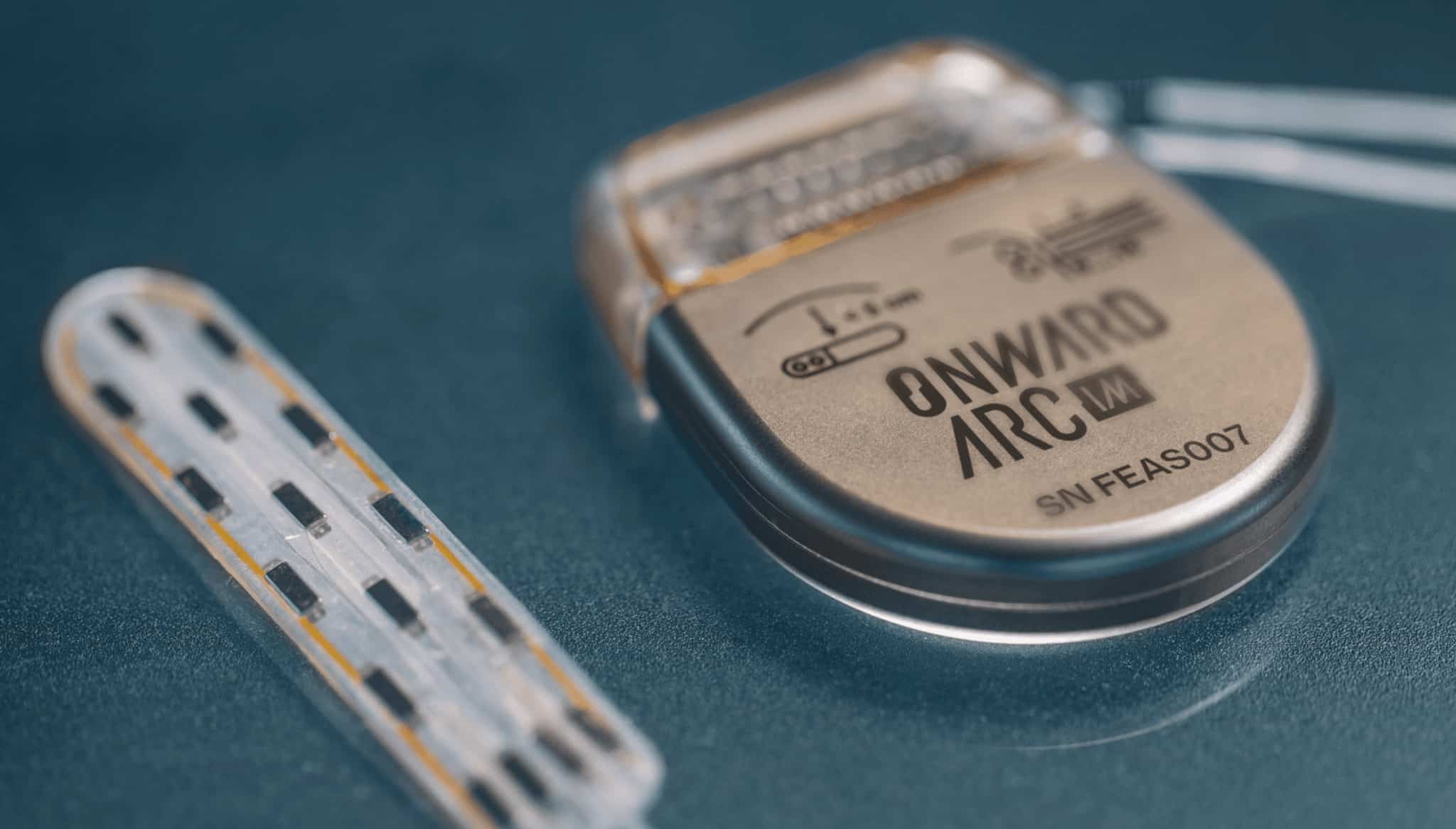
ONWARD is a medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injuries. ONWARD’s work builds on more than a decade of basic science and preclinical research conducted at the world’s leading neuroscience laboratories. ONWARD’s ARC Therapy, which can be delivered by implantable (ARC-IM) or external (ARC-EX) systems, is designed to deliver targeted, programmed spinal-cord stimulation to restore movement and other functions in people with spinal cord injury, ultimately improving their quality of life.
ONWARD has received three Breakthrough Device Designations from the U.S. FDA encompassing both ARC-IM and ARC-EX. ARC-EX is an external, non-invasive platform consisting of a wearable stimulator and wireless programmer. Positive top line data were reported in September 2022 from the company’s first pivotal study, called Up-LIFT, evaluating the ability of ARC-EX Therapy to improve upper extremity strength and function. The company is now preparing marketing approval submissions for the U.S. and Europe. ARC-IM consists of an implantable pulse generator and lead that is placed near the spinal cord. The company completed its first-in-human use of the ARC-IM neurostimulator in May 2022.
ONWARD is headquartered at the High Tech Campus in Eindhoven, the Netherlands. It has substantial operations in Lausanne, Switzerland, and a growing U.S. presence in Boston, Massachusetts. For additional information about the company, please visit ONWD.com. To access our 2022 Financial Calendar, please visit IR.ONWD.com.
For Company Enquiries:
For Media Enquiries:
MC Services AG
US: Laurie Doyle, P: +1 339 832 0752
Europe: Dr. Johanna Kobler, Katja Arnold, Kaja Skorka P: +49 89 210 228 0,
For Investor Enquiries:
Disclaimer
Certain statements, beliefs, and opinions in this press release are forward-looking, which reflect the Company or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve several risks, uncertainties, and assumptions that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, changes in demand, competition, and technology, can cause actual events, performance, or results to differ significantly from any anticipated development. Forward-looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions, or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.