Confo Therapeutics Appoints Dieter Weinand, Former CEO of Bayer Pharmaceuticals, as Independent Chairman
Confo Therapeutics Appoints Dieter Weinand, Former CEO of Bayer Pharmaceuticals, as Independent Chairman
Ghent, Belgium – August 29, 2023 – Confo Therapeutics, a leader in the discovery of medicines targeting G-protein coupled receptors (GPCRs), today announced the appointment of Dieter Weinand as Independent Chairman of its Board of Directors. Mr. Weinand brings with him a wealth of experience with more than 35 years in the pharmaceutical industry and over 25 years as the leader of geographic and strategic business units and drug commercialization efforts across global organizations, including several years as the President, CEO and Chairman of the Board of Bayer Pharmaceuticals AG. He joins Confo as the company advances its pipeline and continues to leverage its proprietary GPCR drug discovery engine. Mr. Weinand succeeds John Berriman, who is retiring from his current position as Chairman of Confo’s Board of Directors.
“Dieter is an exceptional leader in the pharmaceutical industry with a proven track record across the spectrum of research and development to commercialization, bringing impactful medications to patients. A chairman of his caliber will be invaluable as we continue progressing as an organization with a truly innovative GPCR-targeting technology and a growing pipeline of novel drug candidates,” said Cedric Ververken, CEO of Confo Therapeutics. “On behalf of the Confo team, I sincerely thank John Berriman for his guidance over the past seven years and his contributions to Confo’s growth into a clinical-stage drug development company.”
“Confo is moving on an exciting trajectory, harnessing the momentum of a pivotal agreement with Eli Lilly for its first clinical program, CFTX-1554, and building out a robust pipeline of large and small molecules based on its unique platform,” added Dieter Weinand, Independent Chairman. “I look forward to working together with the Confo team to advance the company through its next stages of growth and to achieve its future strategic business and pipeline objectives.”
Mr. Weinand has had a highly successful career across all areas of pharmaceutical development, commercialization, and international organizational leadership. Most recently, he was Head of Global Primary Care Business at Sanofi where he led the optimization of the company’s portfolios. Before this, he was President, CEO, and Chairman of the Board of Bayer Pharmaceuticals AG, where he was responsible for integrating the R&D, manufacturing, and all commercial and support functions of the healthcare business of the Bayer AG corporation. Mr. Weinand held various senior management positions across international corporations including at Bristol-Meyers Squibb and Pfizer. He has been instrumental in the launch and marketing of some of the industry’s pivotal products including Lipitor®, Neurontin®, Abilify® and Cipro®. Mr. Weinand holds a B.A. in Biology from Concordia College, New York, and an M.S. in Pharmacology and Toxicology from Long Island University, New York. Mr. Weinand is Chairman of the Board of Nasdaq-listed Replimune (REPL), FORE Biotherapeutics, and ZielBio, among others.
About Confo Therapeutics
Confo Therapeutics is the only GPCR company with a proprietary discovery engine that precisely targets desired GPCR conformations. This unique capability allows us to unlock a vast untapped potential for the discovery and development of breakthrough medicines. We are advancing a robust pipeline of large and small molecules focused on validated targets in endocrine and metabolic diseases, as well as addressing a broader array of critical unmet medical needs in collaboration with our partners. Our team of accomplished experts is dedicated to advancing our patent protected technology, expanding our capabilities, and building out our pipeline in order to achieve the best possible therapeutic outcomes for patients. For more information, visit www.confotherapeutics.com
For more information, please contact:
Confo Therapeutics
Dr. Cedric Ververken, CEO
+ 32 (0) 9 396 74 00
Email:
Trophic Communications
Valeria Fisher or Desmond James
+49 175 8041816 or +49 (0) 1516 7859086
Email:
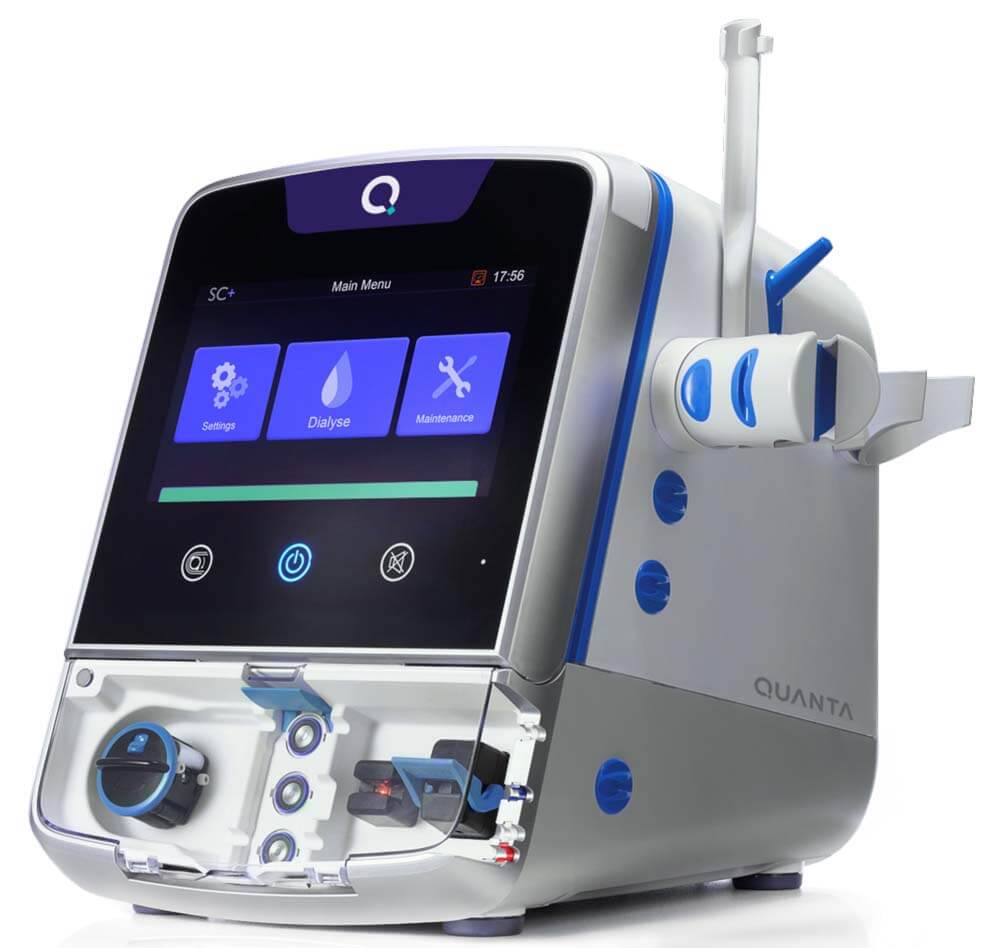
Quanta™ Announces Enrollment Completion in Home Run Study
Quanta™ Announces Enrollment Completion in Home Run Study
Multi-center trial assesses efficacy and safety of the Quanta Dialysis System
BEVERLY, Massachusetts – August 17, 2023 – Quanta Dialysis Technologies® today announced that it has completed enrollment of its Home Run study for at-home hemodialysis. The Home Run study is a prospective, multi-center, open-label trial to assess the efficacy and safety of the Quanta Dialysis System for home hemodialysis. Results are expected to be announced in the second half of 2023.
“People with end-stage kidney disease on hemodialysis are currently limited as to options available. Although frequent home hemodialysis is proven to deliver superior outcomes, for many, in-center dialysis treatment three times a week is the only viable option,” said Quanta Chief Medical Officer, Dr. Paul Komenda, MHA, FRCPC, FASN. “We hope that FDA clearance of an additional home hemodialysis device will contribute to more patients having access to regular care in their home environment. Enrollment completion is the first step forward in a potential new alternative for people with this life-threatening disease.”
About the Home Run Study
The Home Run study is establishing non-inferiority in safety and efficacy when the Quanta Dialysis System is used in the self-care home environment with a care partner compared to a hemodialysis facility. Following enrollment, study participants begin hemodialysis treatments using the system on a prescription of four-hour treatments, three times per week for a minimum of four weeks. During this time, both patients and their caregivers will undergo extensive training and competency sign off on all aspects of safely administering hemodialysis treatment in the home. Upon completion of training and a one-week transition period, participants will perform home treatment four times per week for three-and-one-half hours per treatment for eight weeks.
About End-Stage Kidney Disease (ESKD)
End-stage kidney disease is the final stage of chronic kidney disease, where kidney function has declined enough to the point where the kidneys can longer function on their own. With fully functioning kidneys, waste and excess fluids from the blood are filtered and then excreted in urine. For those with ESKD, the kidneys lose their filtering abilities and dangerous levels of fluid, electrolytes and waste build up in the body. Approximately 800,000 people in the United States are living with ESKD, 71% of which rely on dialysis to manage the disease, while the remaining 29% have a functioning kidney transplant.
About the Quanta Dialysis System
The Quanta Dialysis System is a compact, easy-to-use hemodialysis device FDA-cleared for use in patients with acute and/or chronic renal failure, with or without ultrafiltration, in an acute or chronic care facility. It is also CE-mark approved for use in center or at-home in the UK by adult patients with AV fistula or graft or central venous catheter, and who have an estimated dry weight of greater than 40kg.
About Quanta Dialysis Technologies
Quanta Dialysis Technologies is committed to making dialysis accessible to every patient in every setting with its Quanta Dialysis System. As a portable device with performance comparable to larger, traditional machines, the Quanta Dialysis System is a modular and powerful solution that provides the clinical versatility needed to deliver dialysis care across multiple settings. With a simple-to-use and intuitive user interface, it is designed to be operated by a broad range of users to bring dialysis directly to patients.
The Quanta Dialysis System is commercially available in the United Kingdom for home and
hospital use and in the United States, it is FDA-cleared (K222067) for use in chronic and acute care settings. It is not cleared for home or nocturnal use in the United States.
To learn more about Quanta and its products, visit quantadt.com.
###
Media Contact:
Melinda Freson
Immatics Initiates Phase 1/2 Clinical Trial to Evaluate PRAME TCR Bispecific IMA402 in Patients with Advanced Solid Tumors
Immatics Initiates Phase 1/2 Clinical Trial to Evaluate PRAME TCR Bispecific IMA402 in Patients with Advanced Solid Tumors
TCER® IMA402 is the first next-generation, half-life extended TCR Bispecific targeting PRAME to enter the clinic Patient enrollment for IMA402 Phase 1/2 trial underway The trial will evaluate safety, tolerability, and anti-tumor activity of IMA402 in patients with recurrent and/or refractory solid tumors First clinical data expected in 2024
Tuebingen, Germany and Houston, Texas, August 10, 2023 – Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, today announced the initiation of a Phase 1/2 clinical trial with its proprietary Bispecific T cell engaging receptor (TCER®) IMA402. IMA402 is the second product candidate in Immatics’ TCER® pipeline of next-generation, half-life extended bispecific molecules to enter clinical development. It targets an HLA-A*02:01-presented peptide derived from PRAME, a clinically established cancer target frequently expressed in a large variety of solid tumors.
The Phase 1/2 clinical trial (NCT05958121) investigates TCER® IMA402 in HLA-A*02:01-positive patients with PRAME-expressing recurrent and/or refractory solid tumors. The dose escalation part of the study is designed as a basket trial in focus indications to accelerate signal finding. Initial focus indications are cutaneous and uveal melanoma, ovarian cancer, lung cancer, uterine cancer and synovial sarcoma, among others.
“The addition of IMA402 to our clinical pipeline is a truly exciting step and aligns with our strategic goal to harness the full potential of PRAME, one of the most promising cancer targets in solid tumors. With our half-life extended format, we believe IMA402 has the potential to be an attractive treatment option by enhancing efficacy, minimizing toxicities, and providing favorable dosing regimen for cancer patients.,” said Cedrik Britten, Chief Medical Officer at Immatics. “We are working with urgency to bring IMA402 to a broad patient population as quickly as possible and look forward to sharing first clinical data in 2024.”
Primary objectives of the IMA402 Phase 1/2 trial are to determine the maximum tolerated dose (MTD) and/or the recommended doses for trial extensions, as well as to characterize safety and tolerability of IMA402. Secondary objectives are to evaluate anti-tumor activity and assess pharmacokinetics of IMA402. The Phase 1a dose escalation will be followed by a Phase 1b dose expansion, with the plan then to initiate a Phase 2 with indication-specific cohorts and/or combination therapies. Immatics has implemented an adaptive design for the dose escalation with the goal of accelerating the clinical development timeline of IMA402. Pharmacokinetics data will be assessed throughout the trial and might provide an early opportunity for adjustment of the treatment interval based on the half-life extended TCER® format. The trial is initially planned to be conducted at approximately 15 sites in Europe, with extension into the US at dose expansion stage. The Phase 1a is designed to enroll approximately 45 patients.
The trial initiation is based on the comprehensive preclinical studies with IMA402 presented at the European Society for Medical Oncology (ESMO) Congress 2022.
TCER® IMA402 is the second Immatics clinical program targeting PRAME, with the first being ACTengine® IMA203, a TCR-T cell therapy which is currently being evaluated in a Phase 1b dose expansion. Both approaches, ACTengine® and TCER®, are distinct therapeutic modalities that Immatics believes to have the potential to provide innovative treatment options for a variety of cancer patient populations with different medical needs.
About IMA402
TCER® IMA402 is a drug candidate owned by Immatics. IMA402 is Immatics’ second TCER® molecule from the bispecifics pipeline and is directed against an HLA-A*02-presented peptide derived from preferentially expressed antigen in melanoma (PRAME), a protein frequently expressed in a large variety of solid cancers, thereby supporting the program’s potential to address a broad cancer patient population. Immatics’ PRAME peptide is present at a high copy number per tumor cell and is homogenously and specifically expressed in tumor tissue. The peptide has been identified and characterized by Immatics’ proprietary mass spectrometry-based target discovery platform, XPRESIDENT®. IMA402 is part of Immatics’ strategy to leverage the full clinical potential of targeting PRAME, one of the most promising targets for TCR-based therapies.
About TCER®
Immatics’ next-generation half-life extended TCER® molecules are antibody-like “off-the-shelf” biologics that leverage the body’s immune system by redirecting and activating T cells towards cancer cells expressing a specific tumor target. The design of the TCER® molecules enables the activation of any T cell in the body to attack the tumor, regardless of the T cells’ intrinsic specificity. Immatics proprietary biologics are engineered with two binding regions: a TCR domain and a T cell recruiter domain. The TCER® format is designed to maximize efficacy while minimizing toxicities in patients. It contains a high-affinity TCR domain that is designed to bind specifically to the cancer target peptide on the cell surface presented by an HLA molecule. The antibody-derived, low-affinity T cell recruiter domain is directed against the TCR/CD3 complex and recruits a patient’s T cells to the tumor to attack the cancer cells. With a low-affinity recruiter aiming for optimized biodistribution and enrichment of the molecule at the tumor site instead of the periphery, TCER® are engineered to reduce the occurrence of immune-related adverse events, such as cytokine release syndrome. In addition, the TCER® format consists of an Fc-part conferring half-life extension, stability, and manufacturability. TCER® are “off-the-shelf” biologics and thus immediately available for patient treatment. They can be distributed through standard pharmaceutical supply chains and provide the opportunity to reach a large patient population without the need for specialized medical centers.
– END –
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
Immatics intends to use its website www.immatics.com as a means of disclosing material non-public information. For regular updates you can also follow us on Twitter, Instagram and LinkedIn.
Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the timing of product candidates and Immatics’ focus on partnerships to advance its strategy are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
| Media and Investor Relations Contact | |
| Eva Mulder or Charlotte Spitz | |
| Trophic Communications | |
| Phone: +31 6 52 33 15 79 | |
| Immatics N.V. | |
| Anja Heuer | Jordan Silverstein |
| Senior Director, Corporate Communications | Head of Strategy |
| Phone: +49 89 540415-606 | Phone: +1 281 810 7545 |
Quanta™ Receives FDA 510(k) Clearance for Expanded Indication of Continuous Renal Replacement Therapies
Quanta™ Receives FDA 510(k) Clearance for Expanded Indication of Continuous Renal Replacement Therapies
Quanta Dialysis System becomes first-in-class to perform three standard-of-care dialysis modalities in one device
BEVERLY, Mass., Aug. 3, 2023 /PRNewswire/ — Quanta Dialysis Technologies®, a medical technology company committed to making kidney care more accessible, today announced that it received U.S. Food and Drug Administration (FDA) 510(k) clearance for an expanded indication of the Quanta Dialysis System, a compact and easy-to-use hemodialysis device, for two modalities of continuous renal replacement therapy (CRRT): continuous venovenous hemodialysis (CVVHD) and slow continuous ultrafiltration (SCUF). Under the new 510(k), the Quanta Dialysis System is the only dialysis device FDA-cleared to provide intermittent hemodialysis (IHD), sustained low efficiency dialysis (SLED) or bagless CRRT which creates dialysate on demand – all in a single machine.
The Quanta Dialysis System was originally designed to serve the more than two million people with end-stage kidney disease (ESKD) worldwide who receive treatment with dialysis or a kidney transplant. The latest addition of its Trinal Kidney Therapy™ (TKT) software provides a treatment solution for critically ill patients diagnosed with acute kidney injury (AKI) who require dialysis. It features dialysate flow rates from 50 to 500 mL/min and treatment times up to 24 hours of continuous delivery.
“This clearance is a true game-changer for acute care settings,” said Quanta Chief Executive Officer, Alejandro Galindo. “Hospitals are often constrained with limited space and nursing staff. The Quanta Dialysis System with TKT software provides an all-in-one solution for hospitals with an intensive care unit (ICU) looking to reduce their device footprint, maximize their operational efficiencies, reduce burden on nurses and substantially lower consumables expenses.”
Mortality for ICU patients with severe AKI who need dialysis has been reported to exceed 50% in patients often requiring intravenous life support to maintain a minimum blood pressure. CRRT offers a slower and gentler alternative to conventional dialysis; continuous dialysis runs 24 hours a day as compared to traditional hemodialysis which occurs over a four-hour period enabling better real time management of volume and biochemistry for patients.
“Critically ill patients that are hemodynamically unstable, such as those with severe AKI, are more challenging to manage in the ICU balancing volume status, inotropic support and ventilation requirements. Because of its slower rate of fluid removal, CRRT may cause less stress for the patient and enable more real-time decision making for clinical teams on a minute-to-minute basis,” said Quanta Chief Medical Officer, Dr. Paul Komenda, MHA, FRCPC, FASN. “However, once the hemodynamic status of the patient has improved, a transition to IHD or SLED may be the best option. A device than can perform all three modalities in one is ideal.”
Quanta is ready to commercialize the Quanta Dialysis System with TKT software and expects to officially launch the product at the 2023 American Society of Nephrology Annual Meeting.
About Quanta Dialysis Technologies
Quanta Dialysis Technologies is committed to making dialysis accessible to every patient in every setting with its Quanta Dialysis System. As a portable device with performance comparable to larger, traditional machines, the Quanta Dialysis System is a modular and powerful solution that provides the clinical versatility needed to deliver dialysis care across multiple settings. With a simple-to-use and intuitive user interface, it is designed to be operated by a broad range of users to bring dialysis directly to patients.
The Quanta Dialysis System is commercially available in the United Kingdom for home and hospital use and in the United States, it is FDA-cleared (K222067) for use in chronic and acute care settings. It is not cleared for home or nocturnal use in the United States.
To learn more about Quanta and its products, visit quantadt.com.