Carisma Therapeutics Presents Promising New Preclinical Data on Engineered Macrophages for the Treatment of Liver Fibrosis at AASLD The Liver Meeting® 2024
Carisma Therapeutics Presents Promising New Preclinical Data on Engineered Macrophages for the Treatment of Liver Fibrosis at AASLD The Liver Meeting® 2024
New preclinical results support the anti-fibrotic potential of engineered macrophages in multiple fibrosis models
Engineered TIM4-expressing macrophages correct defective efferocytosis in MASH, demonstrating potent anti-fibrotic activity
PHILADELPHIA, Nov. 17, 2024 /PRNewswire/ — Carisma Therapeutics Inc. (Nasdaq: CARM) (“Carisma” or the “Company”), a clinical-stage biopharmaceutical company focused on discovering and developing innovative immunotherapies, today presented promising preclinical data on engineered macrophages for treating liver fibrosis at the American Association for the Study of Liver Diseases (AASLD) The Liver Meeting® 2024. These results underscore the pre-clinical efficacy of Carisma’s engineered macrophages in multiple liver fibrosis models and offer a novel, off-the-shelf potential treatment option for patients with fibrotic liver disease including advanced metabolic dysfunction-associated steatohepatitis (MASH).
Liver fibrosis is a central late-stage pathway in multiple liver diseases, including MASH, acute liver injury, primary sclerosing cholangitis, primary biliary cholangitis, and others. Treatment options remain limited for advanced liver disease patients. Liver disease is characterized by defective efferocytosis (an anti-inflammatory process by which macrophages clear dead hepatocytes), activation of hepatic stellate cells which leads to collagen accumulation, and chronic inflammation.
New preclinical results demonstrate that macrophages can be genetically engineered to target specific key pathways underlying liver disease with factors including TIM4 (restores efferocytosis), relaxin (inhibits hepatic stellate cell activation), and IL10 (reduces inflammation). Notably, a single dose of macrophages expressing TIM4, alone or together with relaxin, significantly reduced liver fibrosis and hepatic stellate cell activation in the translationally relevant choline-deficient, L-amino acid-defined, high-fat diet (CDAHFD) MASH model. The engineered macrophages were well tolerated and outperformed non-engineered cells in all models.
“We are pleased to present compelling preclinical data supporting the therapeutic potential of our engineered macrophages to address a critical unmet need in liver fibrosis, which is found in advanced stages of MASH,” said Michael Klichinsky, PharmD, PhD, Co-founder and Chief Scientific Officer of Carisma. “These data underscore the efficacy of our engineered macrophages as a differentiated, off-the-shelf approach for treating advanced liver fibrosis. Based on these promising findings, we are committed to advancing our liver fibrosis program.”
Carisma expects to nominate a development candidate for its liver fibrosis program in the first quarter of 2025.
The poster presented at AASLD 2024 is now available online in the “Publications” section of Carisma’s website at https://carismatx.com/technology/publications/
About Carisma Therapeutics
Carisma Therapeutics Inc. is a clinical-stage biopharmaceutical company focused on utilizing our proprietary macrophage and monocyte cell engineering platform to develop transformative immunotherapies to treat cancer and other serious diseases. We have created a comprehensive, differentiated proprietary cell therapy platform focused on engineered macrophages and monocytes, cells that play a crucial role in both the innate and adaptive immune response. Carisma is headquartered in Philadelphia, PA. For more information, please visit www.carismatx.com.

Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to, anticipated discovery, preclinical and clinical development activities for Carisma’s product candidates, the potential safety, efficacy, benefits and addressable market for Carisma’s product candidates, and clinical trial results for Carisma’s product candidates. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. The words “believes,” “anticipates,” “estimates,” “plans,” “expects,” “intends,” “may,” “could,” “should,” “potential,” “likely,” “projects,” “continue,” “will,” “schedule,” and “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are predictions based on the Company’s current expectations and projections about future events and various assumptions. Although Carisma believes that the expectations reflected in such forward-looking statements are reasonable, Carisma cannot guarantee future events, results, actions, levels of activity, performance or achievements, and the timing and results of biotechnology development and potential regulatory approval is inherently uncertain. Forward-looking statements are subject to risks and uncertainties that may cause Carisma’s actual activities or results to differ significantly from those expressed in any forward-looking statement, including risks and uncertainties related to Carisma’s ability to advance its product candidates, the receipt and timing of potential regulatory designations, approvals and commercialization of product candidates, clinical trial sites and our ability to enroll eligible patients, supply chain and manufacturing facilities, Carisma’s ability to maintain and recognize the benefits of certain designations received by product candidates, the timing and results of preclinical and clinical trials, Carisma’s ability to fund development activities and achieve development goals, Carisma’s ability to protect intellectual property, and other risks and uncertainties described under the heading “Risk Factors” in Carisma’s Annual Report on Form 10-K for the year ended December 31, 2023, its Quarterly Reports on Form 10-Q and other documents that Carisma files from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this press release, and Carisma undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof, except as may be required by law.
Carisma Unveils Promising Pre-Clinical Data on Anti-GPC3 In Vivo CAR-M Therapy for Hepatocellular Carcinoma
Carisma Unveils Promising Pre-Clinical Data on Anti-GPC3 In Vivo CAR-M Therapy for Hepatocellular Carcinoma
New findings showcase the potential of in vivo CAR-M technology as an effective, off-the-shelf treatment for hepatocellular carcinoma (HCC)
“The data demonstrate our ability to generate anti-GPC3 CAR-M cells directly in vivo using mRNA/LNP technology, leading to significant tumor reduction in translationally relevant pre-clinical models,” said Michael Klichinsky, PharmD, PhD, Co-Founder and Chief Scientific Officer at Carisma. “This novel off-the-shelf approach offers a promising new strategy for treating hepatocellular carcinoma, and we are eager to advance it toward clinical development.”
“These preclinical data highlights the successful application of our mRNA/LNP platform in enabling in vivo cell therapy,” said Lin Guey, PhD, CSO of Therapeutic Research Ventures, Moderna. “We look forward to further advancing the anti-GPC3 in vivo CAR-M therapy for HCC patients and continuing our collaboration with Carisma to bring innovative treatments to those patients with solid tumors.”
About Carisma Therapeutics
Carisma Therapeutics Inc. is a clinical-stage biopharmaceutical company focused on utilizing our proprietary macrophage and monocyte cell engineering platform to develop transformative immunotherapies to treat cancer and other serious diseases. We have created a comprehensive, differentiated proprietary cell therapy platform focused on engineered macrophages and monocytes, cells that play a crucial role in both the innate and adaptive immune response. Carisma is headquartered in Philadelphia, PA. For more information, please visit www.carismatx.com.

Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to, anticipated discovery, preclinical and clinical development activities for Carisma’s product candidates, the potential safety, efficacy, benefits and addressable market for Carisma’s product candidates, and clinical trial results for Carisma’s product candidates. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. The words “believes,” “anticipates,” “estimates,” “plans,” “expects,” “intends,” “may,” “could,” “should,” “potential,” “likely,” “projects,” “continue,” “will,” “schedule,” and “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are predictions based on the Company’s current expectations and projections about future events and various assumptions. Although Carisma believes that the expectations reflected in such forward-looking statements are reasonable, Carisma cannot guarantee future events, results, actions, levels of activity, performance or achievements, and the timing and results of biotechnology development and potential regulatory approval is inherently uncertain. Forward-looking statements are subject to risks and uncertainties that may cause Carisma’s actual activities or results to differ significantly from those expressed in any forward-looking statement, including risks and uncertainties related to Carisma’s ability to advance its product candidates, the receipt and timing of potential regulatory designations, approvals and commercialization of product candidates, clinical trial sites and our ability to enroll eligible patients, supply chain and manufacturing facilities, Carisma’s ability to maintain and recognize the benefits of certain designations received by product candidates, the timing and results of preclinical and clinical trials, Carisma’s ability to fund development activities and achieve development goals, Carisma’s ability to protect intellectual property, and other risks and uncertainties described under the heading “Risk Factors” in Carisma’s Annual Report on Form 10-K for the year ended December 31, 2023, its Quarterly Reports on Form 10-Q and other documents that Carisma files from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this press release, and Carisma undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof, except as may be required by law.
Investors:
Shveta Dighe
Head of Investor Relations
Media Contact:
Julia Stern
(763) 350-5223
This information is being distributed to you by: Carisma Therapeutics Inc.
3025 Market Street, Ste 140, Philadelphia, PA, 19104,
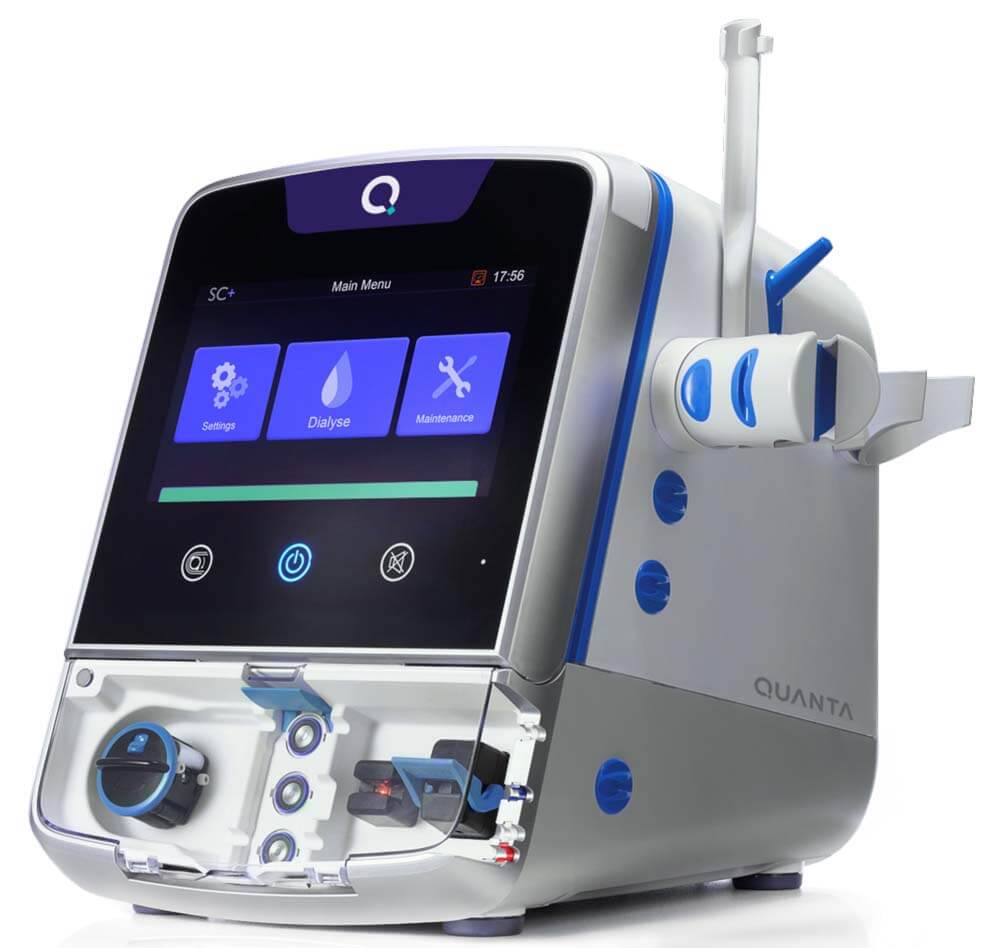
QUANTA™ DIALYSIS SYSTEM RECEIVES FDA CLEARANCE FOR HOME HEMODIALYSIS
QUANTA™ DIALYSIS SYSTEM RECEIVES FDA CLEARANCE FOR HOME HEMODIALYSIS
BEVERLY, Mass., Nov. 4, 2024 /PRNewswire/ — Award-winning medical technology innovator, Quanta Dialysis Technologies®, has confirmed the US Food and Drug Administration (FDA) has given 510(k) clearance for the use of its Quanta Dialysis System in the home setting. With this clearance, Quanta becomes the only company to offer a high dialysate flow (500 mL/min) system across the entire care continuum for end stage renal disease (ESRD) patients.
This significant step in Quanta’s US commercialization efforts follows another recent milestone in which the company received clearance for the first, and only, FDA-cleared device able to perform intermittent hemodialysis (IHD), sustained low efficiency dialysis (SLED), and CRRT (CVVHD and SCUF) without any need for bags.
Based on 2022 data from the United States Renal Data System, of the 550,000 prevalent ESRD patients in the country on dialysis therapy, just 2.4% receive home hemodialysis (HHD). The Quanta Dialysis System (previously known as the SC+ Hemodialysis System) addresses this gap by providing patients access to a high-flow system in the home, the same offering as routinely provided in hospitals, post-acute facilities and in-center clinics.
“The 510(k) clearance embodies the hard work and determination of our exceptional team at Quanta,” said Quanta Chief Executive Officer Alejandro Galindo. “We recruited and executed this pivotal trial during the height of COVID-19 and have now achieved an FDA clearance few other products have obtained. As the US continues to evolve towards value-based care models, the focus for patients with chronic diseases is on minimizing complications and re-admissions.
“Our product is designed to help those patients seamlessly transition to the home and remain there as long as possible. We are currently planning our home launch with customers who have successfully implemented Quanta into acute and sub-acute settings first.”
Decades-Long Troubles with HHD Adoption
Across the US more than 70% of dialysis facilities are currently not certified to offer HHD, and almost half of those with certification have no active HHD patients. Much of this is attributed to the lack of FDA-cleared technologies, alongside challenges associated with the cost-of-therapy and patient ‘burnout’, caused by dialyzing with low flow technologies requiring frequent treatments.
“Many patients prefer the flexibility of home hemodialysis – a flexible schedule, no commute, and with more frequent therapies they feel better and need less medication,” said Quanta Chief Medical Officer Dr. Paul Komenda, MHA, FRCPC, FASN. “Expanding treatment into the home is something we are constantly asked about by clinicians, advocacy groups, providers and patients. One of our major commitments as a business is to make kidney care more accessible, and with an easy-to-train, easy-to-maintain device that accommodates a high-flow HD prescription, Quanta is poised to become the best option for the home.”
Encouraging Clinical Trial Results
An FDA Investigational Device Exemption (IDE) trial of the Quanta Dialysis System was completed in October 2023, including a multi-center, open-label assessment of efficacy and safety. The trial saw 32 patients receive standard in-center hemodialysis while training to use the Quanta Dialysis System, before transitioning to perform HHD four times per week for eight weeks. The study demonstrated the device to be safe and effective, with more than 90% of patients electing to continue using the device after completing the trial.
Study results were presented at the American Society of Nephrology meeting in 2023 and the manuscript is now under review at the Clinical Journal of the American Society of Nephrology.
“Our IDE study has clearly demonstrated best in class dialysis adequacy and high patient satisfaction. Our device gives sufficient dialysis in 3x per week therapy and the option of better outcomes with higher frequency,” added Dr. Komenda.
Notes for editors:
About the Quanta Dialysis System:
As a compact device with performance comparable to larger traditional machines, the Quanta Dialysis System provides the clinical versatility needed to deliver kidney replacement therapy across multiple care settings. With an intuitive user interface and only once-weekly hot rinse requirement, the device is designed to be operated by a broad range of users to bring dialysis directly to patients.
The Royal Academy of Engineering announced Quanta Dialysis Technologies as the recipient of the 2022 MacRobert Award (the UK’s longest running and most prestigious award for UK engineering innovation), joining the ranks of Rolls-Royce, Arup and Raspberry Pi.
To learn more about Quanta and its products, visit www.quantadt.com.
The SC+ Hemodialysis System is now known as the Quanta Dialysis System.
Indications for Use – 510(k) K242269
The SC+ Hemodialysis System is indicated for use in patients with acute and/or chronic renal failure, with or without ultrafiltration, in an acute or chronic care facility. Treatments must be administered under physician’s prescription, by a trained clinician who is competent in the use of the device. The SC+ Hemodialysis System is also indicated for use in the home by trained patients in tandem with a trained care partner.
The SC+ Blood Tube Set is a single use, disposable arterial and venous bloodline set intended to provide extracorporeal access during hemodialysis. The Blood Tubeset is compatible only with the SC+ Hemodialysis System.
Photo: https://mma.prnewswire.com/media/2548064/Quanta_Dialysis_Technologies.jpg
Logo: https://mma.prnewswire.com/media/2548121/Quanta_Dialysis_Technologies_Logo.jpg
SOURCE Quanta Dialysis Technologies