Beren Therapeutics Announces FDA Acceptance of its New Drug Application for Adrabetadex in Infantile-Onset Niemann Pick Disease Type C
Beren Therapeutics Announces FDA Acceptance of its New Drug Application for Adrabetadex in Infantile-Onset Niemann Pick Disease Type C
- Accepted for priority review with Prescription Drug User Fee Act (PDUFA) action date of August 17, 2026
- If approved, adrabetadex would be the only therapy to directly target the underlying pathophysiology of Niemann-Pick disease type C (NPC)
- Comprehensive NDA application includes data showing long-term survival benefit, slowed disease progression, confirmatory biomarkers and nonclinical evidence
THOUSAND OAKS, Calif. – February 23, 2026 – Beren Therapeutics P.B.C.®, the parent company of Mandos LLC® and leader in cholesterol trafficking biology and cyclodextrin-based therapeutics, today announced that the U.S. Food and Drug Administration (FDA) has accepted for review its New Drug Application (NDA) for adrabetadex, an investigational cyclodextrin therapy designed to increase intracellular cholesterol trafficking in Niemann-Pick disease type C (NPC). If approved, adrabetadex would represent a first-in-class, disease-modifying approach to treat infantile-onset NPC, a rare and rapidly fatal pediatric neurodegenerative disorder. The FDA assigned adrabetadex a Prescription Drug User Fee Act (PDUFA) target action date of August 17, 2026.
“The FDA’s acceptance of our NDA submission for review is an important milestone, taking us another step closer to a new treatment option for children living with infantile-onset NPC,” said Jason Camm, Chief Executive Officer of Beren Therapeutics P.B.C. “The FDA’s granting of a Priority Review further reinforces the high unmet need in infantile-onset NPC. Our rigorous and comprehensive NDA package incorporated feedback from the FDA and the NPC community, and we remain focused on advancing our application. We are grateful to the patients, families, clinicians and advocates who made this submission possible.”
The NDA for adrabetadex is supported by data demonstrating a clinically meaningful survival benefit in infantile-onset patients. This evidence is from an externally controlled analysis intended to serve as a single adequate and well-controlled study. The comprehensive submission also includes data showing slowed disease progression, confirmatory biomarker and nonclinical findings and patient experience narratives.
Key findings were recently presented at WORLDSymposium 2026, the largest annual scientific meeting in the field of lysosomal storage disease, including survival data in infantile-onset NPC: a 71% reduction in the risk of mortality in adrabetadex-treated patients compared with matched external controls (HR 0.289; 95% CI, 0.141-0.593; P < 0.0001).
“Infantile-onset NPC is the fastest progressing subtype and has limited treatment options,” said Elizabeth Berry-Kravis, M.D., Ph.D., professor of Pediatrics at Rush University Medical Center and principal investigator of the Expanded Access Program. “The approval of adrabetadex would mark the first time we have a disease-modifying therapeutic option, which is urgently needed for these young children and their families.”
Beren previously announced that in 2025 the FDA granted adrabetadex Breakthrough Therapy Designation, a status that accelerates the development of drugs for serious or life-threatening conditions when early evidence suggests a substantial improvement over existing therapy.
About Niemann-Pick Disease Type C
Niemann-Pick disease type C (NPC) is a rare, autosomal-recessive, severe, neurodegenerative disorder caused by pathologic variants in the NPC1 (~95% of cases) or NPC2 genes, leading to impaired cholesterol trafficking resulting in progressive neurological decline and premature death. Infantile-onset NPC refers to NPC in infants and children who first experience neurological symptoms between 0 and 6 years of age. Earlier neurological onset is associated with more rapid progression and poorer prognosis, with mean survival of ~5.6 years for early infantile-onset (age of neurological onset <2 years) and ~13.4 years for late-infantile onset (2 to <6 years).
About Adrabetadex
Adrabetadex is a proprietary mixture of 2-hydroxypropyl-β-cyclodextrin isomers under investigation as a treatment for Niemann-Pick disease type C (NPC). The data suggest that by re-establishing intracellular cholesterol trafficking, adrabetadex is designed to directly address the underlying pathology of NPC. Data from clinical trials and expanded access programs suggest that adrabetadex is generally well tolerated. The main adverse events associated with adrabetadex include hearing impairment that can be managed with hearing aids when necessary, and post-dose fatigue and/or ataxia. Adrabetadex has not been approved by the FDA or any other health authority at this time.
About Beren Therapeutics P.B.C.
Beren Therapeutics P.B.C. is a founder-led, clinical-stage biotechnology company pioneering the discovery, development and commercialization of cyclodextrin-based therapeutics for conditions characterized by defective cholesterol trafficking. Beren and its subsidiary Mandos LLC are committed to the development of adrabetadex for individuals living with Niemann-Pick disease type C (NPC) and have supported the NPC community by providing access to adrabetadex through an Expanded Access Program (EAP).
Beren’s public benefit purpose is to discover, develop and deliver novel therapies that provide optimal benefit for patients, and to do so by integrating the needs of patients, caregivers, clinicians and health systems from the beginning of the development process and maintaining a long-term focus on delivering meaningful therapies and access.
Beren is headquartered in Thousand Oaks, Calif. To learn more about Beren, the adrabetadex program and Beren’s cholesterol-trafficking focused therapeutic strategy, visit the company’s newly launched website or visit Beren’s LinkedIn channel.
Forward-Looking Statements
This press release contains forward-looking statements, including, but not limited to, statements regarding the FDA’s review of the NDA for adrabetadex; the timing and outcome of regulatory review; the potential for adrabetadex to provide clinical benefit or to address the underlying pathology of Niemann-Pick disease type C; the potential for approval or commercialization; the ability to continue providing access through expanded access; and Beren’s plans to expand its pipeline. Actual results may differ materially due to risks and uncertainties including those associated with clinical development, regulatory review, manufacturing, safety and efficacy outcomes and other factors.
Contacts
Media
Campbell O’Connor
Real Chemistry
Seamless Therapeutics Announces Global Research Collaboration with Lilly to Develop Programmable Recombinase-based Therapeutics for Hearing Loss
Seamless Therapeutics Announces Global Research Collaboration with Lilly to Develop Programmable Recombinase-based Therapeutics for Hearing Loss
- Collaboration will advance a next generation gene editing approach by combining Seamless’ expertise in developing highly precise and efficient recombinases with Lilly’s extensive development expertise in genetic hearing disorders
- Companies will leverage Seamless’ recombinase technology to develop therapeutics for defined hearing loss indications
Dresden, Germany and Lexington, MA, January 28, 2026 – Seamless Therapeutics announced today that it has entered into a strategic global research collaboration and licensing agreement with Eli Lilly and Company (“Lilly,”) to develop and commercialize programmable recombinase-based treatments targeting hearing loss indications, using Seamless’ proprietary recombinase platform. The company’s technology performs large, precise DNA insertions in any target gene sequence, and operates independent of the cell’s natural DNA repair pathway.
Under the terms of the agreement, Seamless will design and program site-specific recombinases directed to correct mutations in certain genes of interest related to hearing loss. Lilly will receive an exclusive license to the programmed recombinases to advance through preclinical and clinical drug development and commercialization.
“Lilly is invested in advancing novel treatment approaches for genetic diseases and shares our vision of bringing genetic medicines to patients who currently have limited treatment options. This collaboration is a validation of our gene editing platform and its broad disease-modifying potential,” said Albert Seymour, Ph.D., Chief Executive Officer of Seamless Therapeutics. We look forward to working with our partners at Lilly in our shared goal to transform the outcome for patients with genetic hearing loss. It’s an exciting opportunity to apply our technology to bring treatments to patients with hearing loss and continue to expand the therapeutic potential for programmable recombinases through our proprietary pipeline.”
As part of the agreement, Seamless receives a guaranteed upfront payment and committed research and development funding. In total, Seamless is eligible for over $1.12 billion, including potential development and commercial milestone payments, excluding tiered royalties on successfully marketed drugs. Further details of the agreement have not been disclosed.
Seamless is translating major breakthroughs in programming recombinases, a class of enzymes that have been widely used in scientific research for decades, transforming their accuracy and flexibility, to enable therapeutic gene editing. The company’s unique technology platform allows for site-specific programmable recombinases that are engineered for specificity and activity in order to precisely insert, exchange, invert, or excise DNA fragments in any target gene sequence in the genome. By advancing Seamless’ novel programmable recombinases, this agreement opens the potential for the technology to address a high unmet need in genetic hearing loss.
***
About Seamless Therapeutics
Seamless Therapeutics is changing the paradigm of gene editing through a pioneering approach that has the potential to address unmet medical needs in patients who suffer from severe conditions. Our technology platform unlocks the programming of recombinases, a highly versatile class of enzymes. We are applying our proprietary know-how to develop a pipeline of disease modifying product candidates across a broad spectrum of indications to expand the therapeutic potential of gene editing.
For more information, please contact:
Seamless Therapeutics
Albert Seymour, CEO
Email:
Seamless Therapeutics media inquiries
Trophic Communications
Stephanie May and Eva Mulder
Tel: +49 171 1855682 or +31 6 52 33 15 79
Email:
Immatics Achieves Clinical Proof-of-Concept of its Next-Generation TCR Bispecific (TCER®) Pipeline with Data on IMA402 (PRAME) and IMA401 (MAGEA4/8) and Announces Next Development Steps
Immatics Achieves Clinical Proof-of-Concept of its Next-Generation TCR Bispecific (TCER®) Pipeline with Data on IMA402 (PRAME) and IMA401 (MAGEA4/8) and Announces Next Development Steps
- IMA402 and IMA401 TCR Bispecifics showed favorable tolerability at RP2D as well as deep and durable responses in heavily pre-treated, last-line patients with a range of solid tumors
- IMA402 PRAME Bispecific at RP2D range resulted in a 30% cORR (6/20) across all indications, including 29% (4/14) in melanoma and 2/3 confirmed responses in ovarian carcinoma
- IMA401 MAGEA4/8 Bispecific at ≥1 mg resulted in a 25% cORR (2/8) in head and neck cancer, 29% cORR (2/7) in melanoma and promising clinical activity in sqNSCLC
- Phase 1a dose escalation completed for both trials; data support IMA402 PRAME Bispecific development opportunities in cutaneous melanoma, gynecologic cancers and in combination with IMA401 MAGEA4/8 Bispecific in sqNSCLC
- Phase 1b dose expansion for IMA402 initiated
Houston, Texas and Tuebingen, Germany, November 12, 2025 – Immatics N.V. (NASDAQ: IMTX, “Immatics” or the “Company”), a clinical-stage biopharmaceutical company and the global leader in precision targeting of PRAME, today announced updated Phase 1a dose escalation data from both product candidates in its TCR Bispecifics (TCER®) pipeline, IMA402 PRAME Bispecific and IMA401 MAGEA4/8 Bispecific, as well as next steps for clinical development.
“Our off-the-shelf TCR Bispecifics have a proprietary next-generation format with half-life extension that is designed to combine optimized tolerability and potent anti-tumor activity while supporting patient-convenient dosing,” said Carsten Reinhardt, M.D., Ph.D., Chief Development Officer at Immatics. “We have now achieved clinical proof-of-concept for both product candidates and seen their potential to make a meaningful impact on patients with limited treatment options through deep and durable responses. We look forward to continuing to drive the development of our bispecifics to advance accessible, innovative therapies that can reach more patients and make a lasting difference in cancer care.”
“Today marks the beginning of a new phase for Immatics, expanding our reach beyond cell therapy and establishing a leading position in the TCR Bispecifics field, with a clear commitment to advancing the clinical development of our bispecifics pipeline,” said Harpreet Singh, Ph.D., CEO and Co-Founder of Immatics. “Building on these data, we are excited to evaluate our IMA402 PRAME Bispecific now across multiple targeted cancer patient populations with significant unmet treatment needs and in potentially synergistic combinations. We are especially enthusiastic about the potential for profound benefit by combining IMA402 with IMA401, our MAGEA4/8 Bispecific, in patients with squamous non-small cell lung cancer, a large, highly underserved and difficult-to-treat indication.”
Carsten Reinhardt, M.D., Ph.D., and Harpreet Singh, Ph.D., will present the complete TCR Bispecifics dataset and next development steps during a conference call and webcast today, November 12, at 8:30 am EST/2:30 pm CET. The presentation is accessible on the ‘Events & Presentations’ page on the Investors & Media section of the Company’s website.
For detailed information, please visit: https://investors.immatics.com/news-releases/news-release-details/immatics-achieves-clinical-proof-concept-its-next-generation-tcr
About Immatics TCR Bispecifics (TCER®)
Immatics’ next-generation half-life extended TCER® molecules are antibody-like “off-the-shelf” biologics that leverage the body’s immune system by redirecting and activating T cells towards cancer cells expressing a specific tumor target. The design of the TCER® molecules enables the activation of any T cell in the body to attack the tumor, regardless of the T cells’ intrinsic specificity. Immatics’ proprietary biologics are engineered with two binding regions: a TCR domain and a T cell recruiter domain. The TCER® format is designed to maximize efficacy while minimizing toxicities in patients. It contains a high-affinity TCR domain that is designed to bind specifically to the cancer target peptide on the cell surface presented by an HLA molecule. The antibody-derived, low-affinity T cell recruiter domain is directed against the TCR/CD3 complex and recruits a patient’s T cells to the tumor to attack the cancer cells. With a low-affinity recruiter aiming for optimized biodistribution and enrichment of the molecule at the tumor site instead of the periphery, TCER® are engineered to reduce the occurrence of immune-related adverse events, such as cytokine release syndrome. In addition, the TCER® format consists of an Fc-part conferring half-life extension, stability, and manufacturability. TCER® are “off-the-shelf” biologics and thus immediately available for patient treatment. They can be distributed through standard pharmaceutical supply chains and provide the opportunity to reach a large patient population without the need for specialized medical centers.
About PRAME
PRAME is a target expressed in more than 50 cancers. Immatics is the global leader in precision targeting of PRAME and has the broadest PRAME franchise with the most PRAME indications and modalities. The Immatics PRAME franchise currently includes three product candidates, two therapeutic modalities and a combination therapy that target PRAME: anzu-cel (IMA203) PRAME cell therapy, IMA203CD8 PRAME cell therapy (GEN2), IMA402 PRAME bispecific, anzu-cel in combination with Moderna’s PRAME cell therapy enhancer.
About IMA402 PRAME Bispecific
IMA402 is a molecule from Immatics’ TCR Bispecifics (TCER®) pipeline directed against an HLA-A*02:01-presented peptide derived from PRAME
IMA402 is currently being evaluated in a Phase 1 trial in patients with solid tumors expressing PRAME. IMA402 is part of Immatics’ strategy to leverage the full clinical potential of targeting PRAME, one of the most promising targets for TCR-based therapies.
About IMA401 MAGEA4/8 Bispecific
IMA401 is a molecule from Immatics’ TCR Bispecifics pipeline that targets an HLA-A*02:01-presented peptide derived from two different cancer-associated proteins, melanoma-associated antigen 4 and/or 8 (“MAGEA4/8”). The MAGEA4/8 peptide has been identified and validated by Immatics’ proprietary mass spectrometry-based target discovery platform XPRESIDENT® and is presented at a 5-fold higher target density (copy number per tumor cell) than the MAGEA4 peptide targeted in other clinical trials.
IMA401 is currently being evaluated in a Phase 1 basket trial in patients with MAGEA4/8-positive solid tumors. The MAGEA4/8 peptide has a high prevalence in several solid tumor indications such as head and neck squamous cell carcinoma (HNSCC), squamous cell non-small cell lung cancer (sqNSCLC), as well as melanoma and other solid cancer types.
About Immatics
Immatics is committed to making a meaningful impact on the lives of patients with cancer. We are the global leader in precision targeting of PRAME, a target expressed in more than 50 cancers. Our cutting-edge science and robust clinical pipeline form the broadest PRAME franchise with the most PRAME indications and modalities, spanning TCR T-cell therapies and TCR bispecifics.
Immatics intends to use its website www.immatics.com as a means of disclosing material non-public information. For regular updates, you can also follow us on LinkedIn and Instagram.
Forward-Looking Statements
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or the Company’s future financial or operating performance. For example, statements concerning timing of data read-outs for product candidates, observations from the Company’s clinical trials, the timing, outcome and design of clinical trials, the nature of clinical trials (including whether such clinical trials will be registration-enabling), the timing of IND, CTA or BLA filings, estimated market opportunities of product candidates, the Company’s focus on partnerships to advance its strategy, and other metrics are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “plan”, “target”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in the Company’s Annual Report on Form 20-F and other filings with the Securities and Exchange Commission (SEC). Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. The Company undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
Media
Trophic Communications
Phone: +49 151 74416179
Immatics N.V.
Jordan Silverstein
Head of Strategy
Phone: +1 346 319-3325
Ipsen to acquire ImCheck Therapeutics, expanding its leadership in oncology, strengthening its pipeline
- Acquisition focused on lead clinical-stage program ICT01 in acute myeloid leukemia, where data from the ongoing Phase I/II EVICTION trial showed high treatment response
- ICT01 has the potential to be a new standard of care in combination in first line unfit acute myeloid leukemia, an aggressive blood cancer affecting older adults
- Ipsen to acquire all issued and outstanding shares of ImCheck Therapeutics, for which ImCheck Therapeutics’ shareholders will be eligible to receive a closing purchase price of 350 million euros and downstream payments contingent upon achievement of regulatory and sales-based milestones
PARIS AND MARSEILLE, FRANCE, 22 October 2025 – Ipsen (Euronext: IPN; ADR: IPSEY) and ImCheck Therapeutics today announced they have entered into a definitive share purchase agreement in which Ipsen will acquire all issued and outstanding shares of ImCheck Therapeutics, a private French biotechnology company pioneering next-generation immuno-oncology therapies. The anticipated acquisition is focused on the lead Phase I/II program ICT01 in first line acute myeloid leukemia (AML)3 patients who are ineligible for intensive chemotherapy. ICT01 is a first-in-class monoclonal antibody targeting BTN3A, a key immune-regulatory molecule broadly expressed across cancer, and received Orphan Drug Designations from the U.S. Food and Drug Administration and European Medicines Agency in July 2025.
Many AML patients are unable to tolerate intensive chemotherapy and must rely on lower-intensity options, which often deliver limited and short-lived benefit.2 This high-risk, unfit population continues to face a significant unmet medical need, highlighting the urgency for new therapies that can improve survival and quality of life.
“At completion, the acquisition of ImCheck Therapeutics presents an opportunity for us to expand our pipeline in oncology and reinforces our commitment to deliver transformative therapies to the people who need them most,” said David Loew, CEO, Ipsen. “We feel confident that with the ICT01 promising data combined with Ipsen’s global development and commercialization expertise, we are well positioned to start a Phase IIb/III trial in 2026.”
Interim data (n=45) orally presented at the annual American Society of Clinical Oncology 20251 from the Phase I/II EVICTION trial showed treatment with ICT01 in combination with venetoclax and azacitidine (Ven-Aza) achieved very encouraging high responses. In this single-arm trial, treatment response nearly doubled relative to those seen in historical standard of care data across all molecular subtypes in newly diagnosed patients including sub-types typically less responsive to standard of care (Ven-Aza).2 ICT01 in combination with Ven-Aza was also shown to be well tolerated, underscoring ICT01’s potential as a novel immunotherapy to improve outcomes for patients with AML.
“We are thrilled to become part of Ipsen, a company whose ambition for transformative care matches our commitment to bringing innovative treatments to patients. This transaction recognizes groundbreaking science originating from French academia,” said Pierre d’Epenoux, CEO, ImCheck Therapeutics. “It also highlights the exceptional work the ImCheck team and our partners have achieved to advance the understanding of butyrophilns and gamma delta T cells. Joining Ipsen will help us accelerate ICT01 toward registrational studies and commercialization. I remain grateful to the patients and investors for their contributions to furthering ImCheck’s pioneering science.”
Transaction details
Under the terms of the agreement, through a wholly owned affiliate of Ipsen SAS, shareholders of ImCheck Therapeutics will receive a 350 million euros payment on a cash-free and debt-free basis at closing of the transaction, and deferred payments contingent upon the achievement of specified regulatory approvals and sales-based milestones, for a total potential consideration up to 1 billion euros.
The transaction is expected to close by the end of Q1 2026, subject to fulfilment of customary closing conditions including the expiration or termination of any required regulatory and governmental approvals under French and U.S. regulations.
Advisors
Allen & Overy Shearman (Paris) is acting as legal counsel to Ipsen. Centerview Partners is acting as exclusive financial advisor to ImCheck Therapeutics with Goodwin (London) and Dentons (Paris) acting as legal counsel.
About the EVICTION trial
EVICTION is a first-in-human, dose-escalation (Part 1) and cohort-expansion (Part 2) clinical trial of ICT01 in patients with various advanced relapsed or refractory solid or hematologic cancers that have exhausted standard-of-care treatment options, as well as newly-diagnosed AML. More information on the EVICTION trial can be found at clinicaltrials.gov (NCT04243499).
About ICT01
ICT01 is a humanized, anti-BTN3A (also known as CD277) monoclonal antibody that promotes the recognition and elimination of tumor cells by γ9δ2 T cells, which are responsible for immunosurveillance of malignancy and infections. The three isoforms of BTN3A targeted by ICT01 are overexpressed on many solid tumors (e.g., melanoma, urothelial cell, colorectal, ovarian, pancreatic,and lung cancer) and hematologic malignancies (e.g., leukemia and lymphomas) and are also expressed on the surface of innate (e.g., γδ T cells and NK cells) and adaptive immune cells (T cells and B cells). Binding to BTN3A is essential for the activation of the anti-tumor immune response of γ9δ2 T cells. By altering the conformation of BTN3A, ICT01 promotes this binding, thereby selectivelyactivating circulating γ9δ2 T cells. This leads to migration of γ9δ2 T cells out of the circulation and into the tumor tissue, and triggers a downstream immunological cascade through secretion of proinflammatory cytokines, including but not limited to IFNγ and TNFα, further augmenting the anti-tumor immune response. Anti-tumor activity and efficacy of ICT01 have been shown in patients across several cancer indications.
About Ipsen
We are a global biopharmaceutical company with a focus on bringing transformative medicines to patients in three therapeutic areas: Oncology, Rare Disease and Neuroscience. Our pipeline is fueled by internal and external innovation and supported by nearly 100 years of development experience and global hubs in the U.S., France and the U.K. Our teams in more than 40 countries and our partnerships around the world enable us to bring medicines to patients in more than 100 countries.
Ipsen is listed in Paris (Euronext: IPN) and in the U.S. through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information, visit ipsen.com.
About ImCheck Therapeutics
ImCheck Therapeutics is developing a new generation of immunotherapeutic antibodies targeting butyrophilins, a novel superfamily of immunomodulators. By unlocking the power of γ9δ2 T cells, ImCheck’s innovative approach has the potential to transform treatments across oncology, autoimmune, and infectious diseases.
The lead clinical-stage program, ICT01, has been advancing to late-stage trials, demonstrating a unique mechanism of action that modulates both innate and adaptive immunity. These “first-in-class” activating antibodies may deliver superior clinical outcomes compared to first-generation immunotherapy approaches, in particular in rationale combinations with immune checkpoint inhibitors and immunomodulatory anti-cancer drugs. Additionally, ImCheck’s pipeline compounds are progressing toward clinical development for autoimmune and infectious diseases.
The company benefits from the pioneering research of Prof. Daniel Olive (Institut Paoli Calmettes, INSERM, CNRS, Aix-Marseille University), a global leader in γ9δ2 T cells and butyrophilins, as well as the expertise of a seasoned management team and the commitment of leading French, European and U.S. investors including Kurma Partners, Eurazeo, Bpifrance through its Innobio 2 and Large Venture funds, Andera Partners, Pfizer Ventures, Gimv, EQT Life Sciences, Earlybird, Wellington Partners, Pureos Bioventures, Invus, Agent Capital, Boehringer Ingelheim Venture Fund, Alexandria Venture Investments, and Blood Cancer United (previously LLS)®.
For further information: https://www.imchecktherapeutics.com/
Ipsen Contacts
Investors
Henry Wheeler +33 7766471149
Khalid Deojee +33 666019526
Media
Sally Bain +1 8573200517
Anne Liontas +33 0767347296
ImCheck Contacts
Media
Gretchen Schweitzer (EU and US) +49 1728618540
Céline Voisin (France) +33 (0)6 62 12 53 39
References
- Dumas et al. γ9δ2 T-cell activation with ICT01 combined with azacitidine-venetoclax for older/unfit adults with newly diagnosed AML: preliminary efficacy and dose selection in Phase 1/2 study EVICTION. ASCO 2025. Available here: https://www.ImChecktherapeutics.com/fileadmin/Posters_Prez/ASCO2025-ICT01-AzaVen.pdf
- Kone AS, Ait Ssi S, Sahraoui S, Badou A. BTN3A: A Promising Immune Checkpoint for Cancer Prognosis and Treatment. Int J Mol Sci. 2022;23(21):13424. Published 2022 Nov 3. doi:10.3390/ijms232113424
- Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593-606. doi:10.1016/S0140- 6736(18)31041-9
Disclaimers and/or forward-looking statements
The forward-looking statements, objectives and targets contained herein are based on Ipsen’s management strategy, current views and assumptions. Such statements involve known and unknown risks and uncertainties that may cause actual results, performance or events to differ materially from those anticipated herein. All of the above risks could affect Ipsen’s future ability to achieve its financial targets, which were set assuming reasonable macroeconomic conditions based on the information available today. Use of the words ‘believes’, ‘anticipates’ and ‘expects’ and similar expressions are intended to identify forward-looking statements, including Ipsen’s expectations regarding future events, including regulatory filings and determinations. Moreover, the targets described in this document were prepared without taking into
account external-growth assumptions and potential future acquisitions, which may alter these parameters. These objectives are based on data and assumptions regarded as reasonable by Ipsen. These targets depend on conditions or facts likely to happen in the future, and not exclusively on historical data. Actual results may depart significantly from these targets given the occurrence of certain risks and uncertainties, notably the fact that a promising medicine in early development phase or clinical trial may end up never being launched on the market or reaching its commercial targets, notably for regulatory or competition reasons. Ipsen must face or might face competition from generic medicine that might translate into a loss of market share. Furthermore, the research and development process involves several stages each of which involves the substantial risk that Ipsen may fail to achieve its objectives and be forced to abandon its efforts with regards to a medicine in which it has invested significant sums. Therefore, Ipsen cannot be certain that favorable results obtained during preclinical trials will be confirmed subsequently during clinical trials, or that the results of clinical trials will be sufficient to demonstrate the safe and effective nature of the medicine concerned. There can be no guarantees a medicine will receive the necessary regulatory approvals or that the medicine will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially fromthose set forth in the forward-looking statements. Other risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and healthcare legislation and risks arising from unexpected regulatory or political changes such as changes in tax regulation and regulations on trade and tariffs, such as protectionist measures, especially in the United States; global trends toward healthcare cost containment; technological advances, new medicine and patents attained by competitors; challenges inherent in new-medicine development, including obtaining regulatory approval; Ipsen’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of Ipsen’s patents and other protections for innovative medicines; and the exposure to litigation, including patent litigation, and/or regulatory actions. Ipsen also depends on third parties to develop and market some of its medicines which could potentially generate substantial royalties; these partners could behave in such ways which could cause damage to Ipsen’s activities and financial results. Ipsen cannot be certain that its partners will fulfil their obligations. It might be unable to obtain any benefit from those agreements. A default by any of Ipsen’s partners could generate lower revenues than expected. Such situations could have a negative impact on Ipsen’s business, financial position or performance. Ipsen expressly disclaims any obligation or undertaking to update or revise any forward looking statements, targets or estimates contained in this pressrelease to reflect any change in events, conditions, assumptions or circumstances on which any such statements are based, unless so required by applicable law. Ipsen’s business is subject to the risk factors outlined in its registration documents filed with the French Autorité des Marchés Financiers. The risks and uncertainties set out are not exhaustive and the reader is advised to refer to Ipsen’s latest Universal Registration Document, available on ipsen.com.
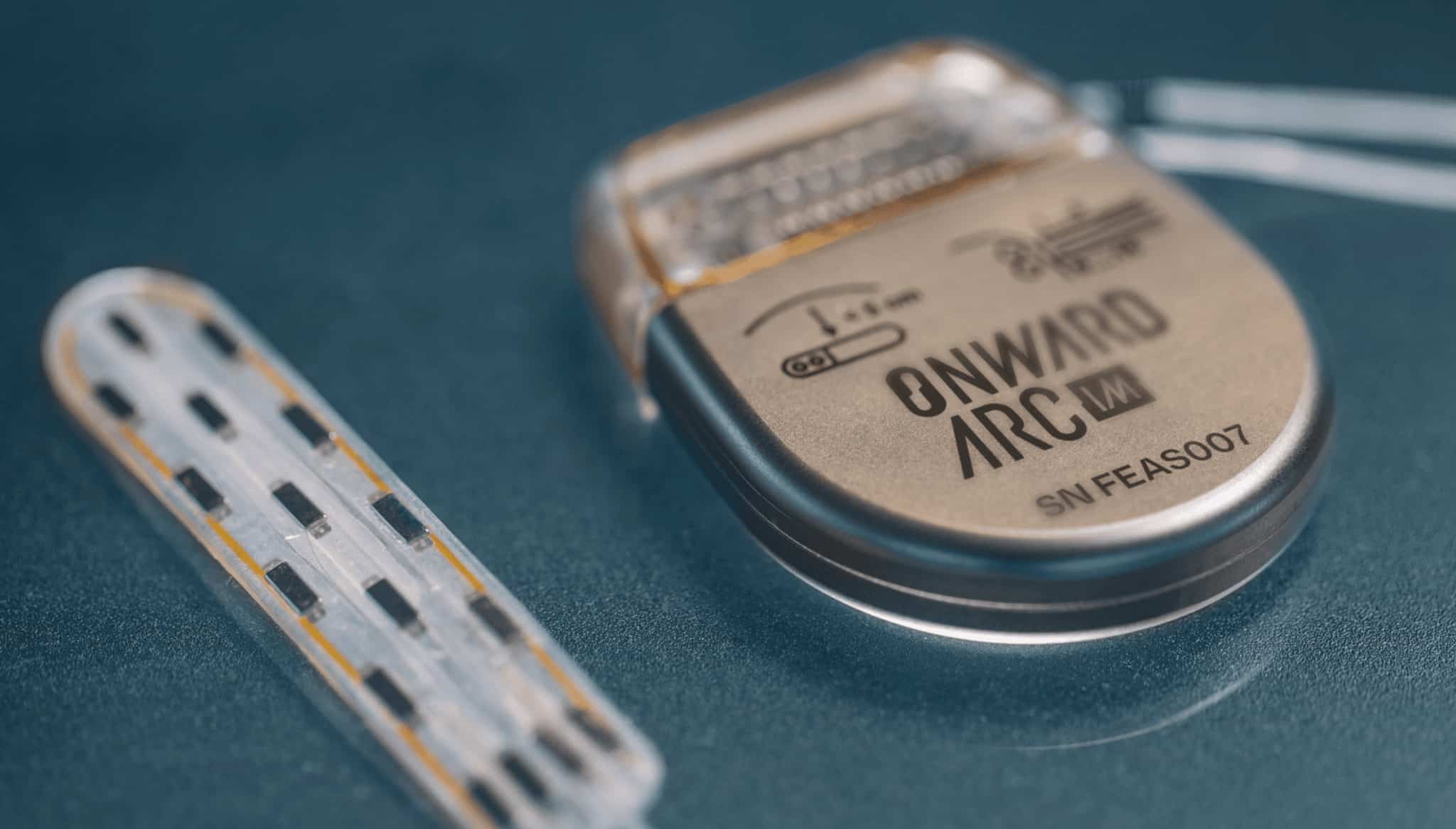
ONWARD Medical Receives CE Mark for ARC-EX, Enabling Commercial Launch of Breakthrough Spinal Cord Stimulation System in Europe
THIS PRESS RELEASE CONTAINS INSIDE INFORMATION WITHIN THE MEANING OF ARTICLE 7(1) OF THE EUROPEAN MARKET ABUSE REGULATION (596/2014)
• The CE Mark certification allows marketing for both clinic and home use
• First commercial sales of the ARC-EX® System in Europe are expected in Q4 2025
• The CE Mark enables commercialization in the European Union and facilitates a streamlined regulatory pathway in other countries, including in the UK and Switzerland
Eindhoven, the Netherlands, September 8, 2025 — ONWARD Medical N.V. (Euronext: ONWD – US ADR: ONWRY), the leading neurotechnology company pioneering therapies to restore movement, function, and independence in people with spinal cord injuries (SCI) and other movement disabilities, today announced it has received CE Mark certification for its ARC-EX System under the European Union Medical Device Regulation (MDR), enabling commercialization in the European Union and certain other countries.
This certification enables the Company to promote the use of the ARC-EX System in conjunction with functional task practice to improve hand strength and sensation in adults with a chronic, non-progressive neurological deficit resulting from an incomplete spinal cord injury (C2-C8 inclusive). The certification allows marketing for both clinic and home use. The ARC-EX System is non-invasive and delivers programmed, transcutaneous electrical spinal cord stimulation via electrodes placed on the back of the neck.
“Hand sensation and strength is a primary recovery target after spinal cord injury. The ARC-EX Therapy opens new doors for the SCI community in Europe, offering opportunities for recovery and care that were previously unavailable," said Dave Marver, Chief Executive Officer of ONWARD Medical. "The CE Markcertification for ARC-EX was awarded far earlier than expected and it gives us great satisfaction to bring this important new therapeutic option to the European spinal cord injury community. We will initiate a phased launch in Europe in the coming weeks, starting with Germany, and cascading to other countries as soon as possible thereafter."
The ARC-EX System is supported by a unique body of clinical evidence. Results of the Up-LIFT pivotal study published in Nature Medicine showed that 90% of study participants improved strength or function, 87% reported improvement in quality of life, and benefits were observed up to 34 years post-injury. The study also reported less spasm frequency, improved sleep quality, and improved upper body sensation and sense of touch. Additionally, results of the investigator-sponsored Pathfinder2 Study, published in Neuromodulation: Technology at the Neural Interface, showed that ARC-EX Therapy, combined with activity-based rehabilitation, delivered significant functional improvements and continued gains in upper body strength, trunk control, and balance after one year of treatment, with no plateau in therapeutic benefit1.
As part of the CE Mark application process, ONWARD Medical achieved its first certification in accordance with the European Medical Device Regulation (MDR), meeting European standards and requirements relating to patient safety, clinical performance, risk management, and post-market surveillance.
Earlier this year, the Company initiated the phased launch of the ARC-EX System in US clinics following US Food and Drug Administration (FDA) clearance. The Company recently reported having met its commercial objective for the first half of 2025, with positive feedback and strong demand from its initial users. The ARC-EX System was selected as a TIME Magazine Best Invention in 2024 and it was also recognized as one of Fast Company's 2025 World Changing Ideas for its potential to transform lives.
About ONWARD Medical
ONWARD Medical is the leading neurotechnology company pioneering therapies to restore movement, function, and independence in people with spinal cord injuries and other movement disabilities. Building on decades of scientific discovery, preclinical research, and clinical studies conducted at leading hospitals, rehabilitation clinics, and neuroscience laboratories, the Company developed ARC Therapy. It has subsequently been awarded 10 Breakthrough Device Designations from the FDA. The Company’s ARC EX® System is cleared for commercial sale in the US and Europe. The Company is also developing an investigational implantable system called ARC-IM®, designed to address several unmet needs including blood pressure instability after spinal cord injury. It can also be paired with a brain-computer interface (BCI) and artificial intelligence (AI) to restore thought-driven movement.
Headquartered in the Netherlands, the Company has a Science and Engineering Center in Switzerland and a US office in Boston, Massachusetts. The Company is listed on Euronext Paris, Brussels, and Amsterdam (ticker: ONWD) and its US ADRs can be traded on OTCQX (ticker: ONWRY). For more information, please visit ONWD.com.
To stay informed about ONWARD’s research studies, technologies, and the availability of therapies in your area, please complete this webform.
For Media Inquiries:
Sébastien Cros, VP Communications
For Investor Inquiries:
Forward-Looking Statements
Certain statements, beliefs, and opinions in this press release are forward-looking, which reflect the Company’s or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve several risks, uncertainties, and assumptions that could cause actual results or events to differ materially from those expressed or implied by the forward looking statements. These risks, uncertainties, and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, delays in regulatory approvals, changes in demand, competition, and technology, can cause actual events, performance, or results to differ significantly from any anticipated development. Forward-looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions, or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.
Trademarks: ONWARD, ARC-EX, ARC-IM, ARC-BCI, and the stylized O-Logo are proprietary and registered trademarks of ONWARD Medical. Unauthorized use is strictly prohibited.
1ARC-EX Indication for Use (EU): The ARC-EX System is intended to deliver programmed, transcutaneous electrical spinal cord stimulation in conjunction with functional task practice in the clinic and with take-home exercises in the home to improve hand sensation and strength in individuals between 18 and 75 years old that present with a chronic (>1 year post-injury), non-progressive neurological deficit resulting from an incomplete spinal cord injury (C2-C8 inclusive).
Other Investigational Products: All other ONWARD Medical devices and therapies including ARC-IM and ARC-BCI are investigational and not available for commercial use.
UroMems Receives Clearance from the FDA and the French ANSM for Initiating Landmark Pivotal Clinical Study of UroActive® Smart Implant to Treat Male Stress Urinary Incontinence
DE approval in both countries follows strong feasibility clinical results in France
GRENOBLE, France and MINNEAPOLIS, July 17, 2025 /PRNewswire/ -- UroMems, a global company developing innovative, mechatronics technology to treat stress urinary incontinence (SUI), received investigational device exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) and French National Agency for the Safety of Medicines and Health Products (ANSM) clearance, enabling the company to begin a first-of-its-kind pivotal clinical trial of the UroActive smart implant to treat stress urinary incontinence (SUI) in men.
This prospective, multicenter trial, called the SOPHIA2 study, will evaluate the safety and efficacy of the UroActive System, the first smart automated artificial urinary sphincter (AUS) for the treatment of SUI. The FDA IDE approval and ANSM clearance follow strong feasibility study results for both women and men in France.
"This marks a key milestone that has been more than a decade in the making, and brings us a significant step closer to delivering the relief from symptoms and return to life that UroActive has the potential to provide patients suffering from SUI," says Hamid Lamraoui, UroMems chief executive officer and co-founder. "UroActive is the first and only smart automated AUS to reach this critical milestone, indicating a new era for millions of people suffering from SUI, while signaling an exciting transition for surgeons treating SUI across the U.S. and Europe."
UroActive is powered by a MyoElectroMechanical System (MEMS). This innovative system is placed around the urethral duct and is controlled based on the patient's activity, without the need for complex manipulation, intending to provide patients with ease of use and a better quality of life than current options.
Co-principal US investigators include Dr. Melissa Kaufman, FPMRS, professor and chief reconstructive surgery at Vanderbilt University in Nashville and Dr. Drew Peterson, FPMRS, professor at Duke University in Durham, NC. "We have seen first-hand the shortcomings of current SUI treatment options for our male and female patients," said Dr. Kaufman on behalf of both co-principal investigators. "That's why we're so excited to be leading the SOPHIA2 trial, as it's showing promise to provide significant improvements in addressing these issues. Based on the feasibility study data we've seen, UroActive has the potential to be a transformational technology."
"We've seen exceptionally strong results for both men and women in France as part of the feasibility clinical study, including over one year with no need for revision nor explant and extremely high praise from patients who had been suffering from SUI for years," said Professor Emmanuel Chartier-Kastler, Urology Chair, Sorbonne University and Pitié-Salpêtrière Hospital in Paris. "We look forward to conducting the pivotal SOPHIA2 study in France in lock step with the U.S. sites."
SUI, or involuntary urinary leakage, affects an estimated 40 million Americans and 90 million Europeans. SUI significantly impacts quality of life, as it can be debilitating, and often leads to depression, low self-esteem and social stigma.
SOPHIA2 study will serve as the basis for UroMems' regulatory submission to the FDA and supports its broader strategy to commercialize UroActive in the U.S. and European markets.
About UroActive
The UroMems technology platform is protected by more than 180 granted patents and is designed to overcome the limitations of current solutions by optimizing safety and performance, patient experience and surgeon convenience. UroActive is the first active implantable electronic artificial urinary sphincter (AUS) that is being developed to compensate for sphincter insufficiency in patients, both men and women, with SUI. It is based on a unique mechatronic platform using embedded smart, digital and robotic systems. UroActive has not received marketing authorization from the FDA and is not available for sale in the United States or in the EU. This project is financially supported by the European Innovation Council and France 2030.
For more information, please visit www.uromems.com.
Media Contact:
Shelli Lissick
651-276-6922
Wellington Partners Co-Leads €84 Million Series B Financing of Nuclidium to advance its Copper-based Radiopharmaceutical Platform
NUCLIDIUM Closes CHF 79 Million (EUR 84 Million) Series B Financing to Advance Clinical Development of its Copper-based Radiopharmaceutical Platform
- Proceeds will fund further clinical development of the company’s true theranostic pipeline and expansion of the global production and manufacturing network for copper-based radiopharmaceuticals.
- Initial clinical data presented at SNMMI 2025 by Dr. Gary Ulaner, MD, PhD show a solid safety profile and potentially improved performance of 61Cu-NuriPro in metastatic prostate cancer imaging.
- The financing round was led by Kurma Growth Opportunities Fund, Angelini Ventures, Wellington Partners, and Neva SGR (Intesa Sanpaolo Group), with participation from DeepTech & Climate Fonds (DTCF), Bayern Kapital, Eurazeo, Vives Partners, NRW.BANK and HighLight Capital, with existing investors.
- Alongside Tony Rosenberg, who recently joined as the Chairman of the Board of Directors, David Meek joins as an additional new Independent Director; Oliver Sartor, MD and Bela Denes, MD join as additional Scientific Advisors.
Basel, Switzerland / Munich, Germany, July 10, 2025 – NUCLIDIUM AG, a clinical-stage radiopharmaceutical company developing a proprietary copper-based theranostic platform, today announced the successful closing of its Series B financing round, raising CHF 79 million (EUR 84 million). The round was led by Kurma Growth Opportunities Fund, Angelini Ventures, Wellington Partners, and Neva SGR (Intesa Sanpaolo Group), with participation from DeepTech & Climate Fonds (DTCF), Bayern Kapital, Vives Partners, Eurazeo, NRW.BANK and HighLight Capital, as well as existing investors. The proceeds will be used to advance the clinical development of NUCLIDIUM’s Copper-61/Copper-67 (61Cu/67Cu) theranostic pipeline across multiple oncology indications. In parallel, the company will expand its production and manufacturing capabilities through a global production network.
NUCLIDIUM’s differentiated platform links tumor-targeting molecules with copper isotopes – Copper-61 for diagnostics and Copper-67 for therapeutics – to address current limitations in radiotheranostics, such as suboptimal clinical efficacy and complex manufacturing. Diagnostic results from initial clinical trials in these indications show superior lesion detection and higher tumor-to-background ratios compared with clinically approved tracers. Initial data were recently presented at SNMMI 2025 by Dr. Gary Ulaner, MD, PhD highlighting a favorable safety profile and potentially improved imaging performance of 61Cu-NuriPro™ compared to current PET imaging standards, suggesting strong clinical promise and broader potential for 61Cu/67Cu theranostic pairing. Early therapeutic data from the two lead compounds, NuriPro™ and TraceNET™, show strong tumor-to-background ratios in metastatic prostate cancer and neuroendocrine tumors including breast cancer.
“NUCLIDIUM is entering the next clinical phases with its lead compounds to diagnose and treat metastatic prostate, neuroendocrine tumors and breast cancer,” said Leila Jaafar, PhD, CEO and Co-Founder of NUCLIDIUM. “Our copper-based radiotheranostics are developed for seamless use in hospital workflows, care delivery and waste management, making these therapies more accessible worldwide. Our groundbreaking next generation copper theranostic platform also allows us to rapidly develop new targets across a wider range of cancers, particularly those highly relevant to women’s health.”
With this financing, NUCLIDIUM will continue expanding its worldwide production and manufacturing network for diagnostics and therapeutics, growing its international team, and strengthening strategic collaborations with hospitals and academic centers, initially across Europe and North America.
In conjunction with the financing round, Daniel Parera, MD, Partner at Kurma Partners, Regina Hodits, PhD, Managing Director at Angelini Ventures, and Liliana Nordbakk, Partner Life Sciences at Neva SGR, will join NUCLIDIUM’s Board of Directors.
“This significant Series B financing reflects the confidence of our investors in NUCLIDIUM’s vision and the transformative potential for the diagnostic and therapeutic industry in oncology and nuclear medicine,” said Tony Rosenberg, Chairman of the NUCLIDIUM Board. “With this backing, we are positioned to accelerate clinical development, broaden patient access globally, and reinforce our commitment to innovation in precision oncology. I am delighted to welcome our new Board and advisory members, whose deep expertise will further strengthen NUCLIDIUM’s leadership in radiopharmaceuticals.”
“NUCLIDIUM’s platform stands out in a rapidly evolving field and will change how radiotheranostic care is delivered. This investment reflects our strong conviction in the future of precision medicine and our belief in NUCLIDIUM’s potential to scale as a next-generation company — an ambition shared across a strong European syndicate,” added Daniel Parera, MD, Partner at Kurma Partners, Regina Hodits, PhD, Managing Director at Angelini Ventures, and Liliana Nordbakk, Partner Life Sciences at Neva SGR for all participating investors.
The Series B financing transaction was advised by VISCHER AG, and Walder Wyss, Switzerland as legal counsels.
About NUCLIDIUM
NUCLIDIUM AG is a clinical-stage biotechnology company pioneering the development of next-generation copper-based radiopharmaceuticals for the diagnosis and treatment of cancer. Leveraging copper isotopes – Copper-61 for diagnostics and Copper-67 for therapeutics – NUCLIDIUM is creating a differentiated platform with the potential to overcome existing limitations in radiotheranostics. The company's operations in Switzerland and Germany combine innovative chemistry, deep clinical expertise, and strategic manufacturing capabilities to deliver scalable, accessible, and clinically superior theranostic solutions to patients worldwide. NUCLIDIUM is committed to expanding the reach and efficacy of radiotheranostics, including addressing critical unmet medical needs in oncology and women’s health.
For more information, please contact:
NUCLIDIUM
Leila Jaafar, PhD, CEO
Email:
Investor/Media Contact NUCLIDIUM
Trophic Communications
Stephanie May
Email:
Phone: +49 171 1855682
CorFlow Therapeutics Announces FDA Approval of the MOCA-II IDE Pivotal Trial to Validate a Novel Heart Attack Care Technology
CorFlow Therapeutics Announces FDA Approval of the MOCA-II IDE Pivotal Trial to Validate a Novel Heart Attack Care Technology
BAAR, Switzerland--(BUSINESS WIRE)-- CorFlow Therapeutics AG (CorFlow), a pioneering company in the field of cardiac care targeting microvascular disease, today announced that the U.S. Food & Drug Administration (FDA) has approved the company’s technology for investigational device exemption (IDE), which allows the pivotal clinical trial to begin at U.S. hospitals. CorFlow will now prepare these clinical trial sites to receive CorFlow systems, undergo training and begin enrolling patients being treated for heart attacks.
The IDE Pivotal Trial, MOCA-II, is intended to prospectively validate the diagnostic accuracy of the proprietary CorFlow CoFl system in determining the presence or absence of microvsacular obstruction (MVO) during a primary PCI procedure. The primary endpoint compares the CoFI diagnostic reading to a reference standard of diagnosis by a cardiac MRI scan. The trial is approved to enroll over 200 STEMI patients at prestigious research institutions in both the United States and Europe.
Having successfully completed the first-in-human MOCA-I trial in 2024, the MOCA-II study is the next critical step to bringing this unique technology into the hands of interventional cardiologists globally for the rapid diagnosis of MVO in heart attack patients. This in turn can enable new treatments and care pathways to the large MVO patient population with high rates of adverse clinical outcomes today. The CorFlow technology is designed to both diagnose MVO, plus serve as a localized drug delivery system for diagnostic and therapeutic agents, which is being researched independently.
According to the US government Centers for Disease Control and Prevention, someone has a heart attack every 30 seconds in the USA, with about 800,000 cases reported annually in the country. Incidence and prevalence are similarly high in Europe. More than half of STEMI heart attack patients are shown to have MVO, and previous research has demonstrated that the presence of MVO is a major driver of adverse events. Currently, there are no technologies approved to diagnose MVO during an acute coronary intervention, and there are no approved therapeutic devices that specifically address MVO in the United States or Europe. Heart attacks and related heart disease remains a leading cause of death and disability worldwide.
Paul Mead, CEO of CorFlow, said “The long history of interventional cardiology and heart attack care breakthroughs - going back over 100 years – is one of the great success stories of medical care progress, but the pioneers and luminaries of the field all agree that the work is unfinished. The majority of acute STEMI survivors have MVO, and current outcomes for these patients are shockingly poor. We aim to bring this issue to light and show you can do something about it. This milestone brings us all one step closer to delivering on the promise to improving care for these people where we know we can do better.”
MOCA-II is being led by world-renowned experts in heart attack care, Dr. Timothy Henry at The Christ Hospital in Cincinnati, Ohio (United States) and Professor Marco Valgimigli at Cardiocentro Ticino Institute, Lugano (Switzerland), who collectively have been published in over 1000 peer-reviewed manuscripts in cardiovascular research.
Dr. Tim Henry said “As an interventional cardiologist involved for decades in managing and researching STEMI patients, I am excited to get going on this pivotal trial with technology that could make such a significant impact to the outcome of our patients. I believe strongly that knowing with high confidence who has MVO at the point of care during a primary PCI procedure can make an immediate difference in how we manage our patients”. Professor Valgimigli added “Having played a significant part in MOCA-I first in human trial, I am thrilled to see the second-generation technology now available for the pivotal trial and am looking forward to contributing further to the scientific understanding of MVO in real time. While the medical community has diverse opinions on how to treat these patients, there is no question that proper diagnosis is the first step we need. I am optimistic that getting the CorFlow technology approved for everyday use by our peer interventional cardiologists can help move the field forward.”
About CorFlow Therapeutics: Headquartered in Baar, Switzerland, with subsidiary operations in both Italy and the United States. The company is venture capital funded with an international VC firm syndicate, most recently with a Series B financing round announced in September 2024. CorFlow aspires to be the leader in diagnostic and therapeutic solutions for restoring healthy microvascular blood flow anywhere in the human body where a critical need exists. Working in close partnership with scientists from the University of Bern, ETH Zurich and the University Hospital Zurich, in a collaboration funded by the Swiss Innovation Agency (Innosuisse), CorFlow continues to explore applications for the unique patented technology.
Media and Scientific Contact
CorFlow Therapeutics AG
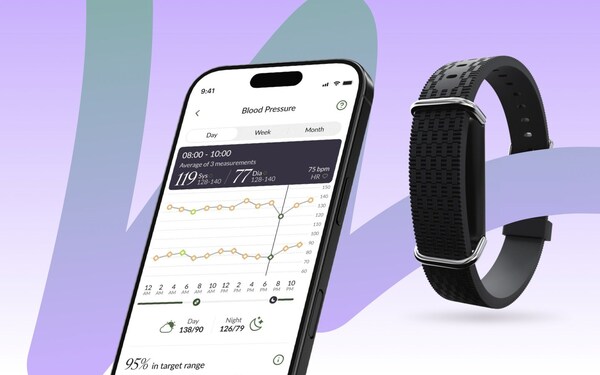
Aktiia secures $42 million in Series B funding round and rebrands to Hilo
Aktiia secures $42 million in Series B funding round and rebrands to Hilo
With fresh funding round, the health tech pioneer continues to work toward managing the world’s blood pressure.
LONDON, May 1, 2025 – Aktiia, a blood pressure intelligence platform, announces an oversubscribed $42 million (over CHF 34 million) Series B funding round, co-led by Earlybird Health and Wellington Partners, with new investors Kfund and naturalX Health Ventures, and participation from existing investors redalpine, Khosla Ventures, Molten Ventures, Translink Capital and Verve Ventures. The investment, which has brought the total financing to date above $100 million, will accelerate the company’s mission to manage the world’s blood pressure.
The latest investment builds on a period of significant momentum for the company during which it has achieved several major milestones, including securing CE marking for its innovative mobile phone camera blood pressure monitoring technology, securing regulatory approvals in Canada, Australia and Saudi Arabia, achieving 76% compounded annual revenue growth, and completing a pivotal clinical trial ahead of its U.S. FDA application submission. This Series B funding will fuel continued product innovation, expansion into new markets and the build out of its blood pressure intelligence platform to support enterprise use cases.
Alongside this funding, Aktiia is introducing its new brand identity: Hilo – a rebrand which reflects the company’s global ambition to make cuffless, clinically validated blood pressure monitoring universally accessible and effortless. The transition to Hilo marks an important milestone in the company’s journey, aligning with its vision of healthier blood pressure for all, while continuing to deliver the trusted technology and reports that have been central to its platform.
With more than 120,000 devices sold, Hilo has already established itself as a stand-out in the health tech space. The company has developed a foundation Machine Learning model specifically designed for blood pressure. The model has been trained on tens of billions of optical signals from real-world users and further refined with hundreds of millions of calibration points, strengthening its position as the leader in the emerging cuffless blood pressure monitoring (CBPM) category.
Raghav “Rags” Gupta, CEO of Hilo, commented:
“This funding round is a testament to the confidence our investors have in Hilo’s groundbreaking technology and our mission to manage the world’s blood pressure via more frequent, convenient and accurate measurements. With billions worldwide suffering from hypertension, only 20% of whom are in control of their blood pressure, the need for innovative, accessible blood pressure monitoring solutions has never been greater. Our rebrand to Hilo represents more than just a name change, it’s a commitment to empowering users around the world with smarter tools to optimise their health. We are grateful to early Aktiia customers for their support and to our investors, new and existing, for their faith.”
Christoph Massner, Principal at Earlybird Health, who will join Hilo’s Board of Directors, commented:
“ Hilo stands at the intersection of medical-grade precision and consumer-centric accessibility. By building on a validated medical device platform and an intuitive, user-friendly design, they bridge the gap between clinical reliability and everyday usability. It’s a rare combination that empowers users to take meaningful control of their health with minimal effort. This is especially critical given the enormous medical need: uncontrolled high blood pressure remains one of the leading risk factors for death worldwide .”
Johannes Fischer, Managing Partner at Wellington Partners, commented:
“We are excited to co-lead this financing and to be backing Hilo’s innovative technology, which provides groundbreaking advancements to the billions of people with elevated bloodpressure today. We believe that real-time and continuous access to blood pressure information will change the way we think about the monitoring and management of one of the most common risk factors to human health.”
ENDS
About Hilo
Aktiia, now trading under Hilo, was established in 2018 to transform how blood pressure is measured and therefore managed. By integrating advanced AI algorithms with extensive datasets, Hilo’s cuffless blood pressure monitoring (CBPM) products offer convenient, valuable reports to give both individuals and healthcare professionals a useful picture of their blood pressure. The company’s foundation model for blood pressure has been trained on billions of optical signals and hundreds of millions of readings across more than 120,000 users, underscoring its leadership in the CPBM category. The company’s multidisciplinary team, composed of experts with extensive experience in biomedical signal processing, has contributed to over 120 peer-reviewed publications and holds more than 35 patents. Headquartered in Switzerland, Hilo continues to expand its global presence, transforming how blood pressure is understood and managed.
The Hilo system by itself is not intended to make diagnoses. Data should always be used in consultation with your healthcare provider.
Website: www.hilo.com
For more information on Hilo, please contact or call 07585 294566
HepaRegeniX completes €21.5 million financing to support clinical advancement of HRX-215 for liver regeneration
HepaRegeniX completes €21.5 million financing to support clinical advancement of HRX-215 for liver regeneration
Tuebingen, Germany, April 15, 2025 – HepaRegeniX GmbH (“HepaRegeniX”), a clinical-stage company advancing novel therapies to treat acute and chronic liver diseases, today announced the second closing and completion of a €21.5 million financing round with the addition of Wellington Partners to its investor syndicate. HepaRegeniX plans to use the proceeds to complete its ongoing Phase Ib trial and to advance the Phase IIa clinical trial for HRX-215, the company’s lead clinical candidate in liver regeneration.
“We continue to make significant progress in the clinical evaluation of our lead candidate, HRX 215, and the additional investment is a strong acknowledgment of our achievements and the impressive safety results of HRX-215 in the completed Phase I trials,” said Elias Papatheodorou, Chief Executive Officer at HepaRegeniX. “HepaRegeniX has brought to light the significant therapeutic potential of Mitogen-Activated Protein (MAP) Kinase Kinase 4 (MKK4) in multiple indications of high medical need, and we remain committed to making a real difference in the lives of patients that need liver resections due to tumors, for patients in need of a liver transplant, and for patients with chronic and acute liver diseases. As we gain momentum in our clinical development strategy, we appreciate the continued support of our existing investors and the support of Wellington Partners at this important stage in our journey.”
“Wellington Partners is very excited to join and further strengthen the HepaRegeniX investor base in this financing round,” commented Dr. Rainer Strohmenger, Managing Partner at the Munich based life science venture capital fund. “HepaRegeniX’ approach is highly differentiated and has demonstrated efficacy in several in vivo models for liver diseases with extraordinarily high unmet medical need. We look forward to supporting the generation of meaningful clinical efficacy data in human patients in the near future.”
HRX-215 is a first-in-class, orally available small molecule inhibitor designed to target Mitogen Activated Protein (MAP) Kinase Kinase 4 (MKK4), a master regulator of liver regeneration. By selectively inhibiting MKK4, HRX-215 enhances the liver’s natural ability to regenerate. Extensive preclinical studies in mice and pigs have demonstrated that HRX-215 enhances liver regeneration of healthy and diseased livers. Impressively, HRX-215 helps prevent liver failure after extended liver resection. A Phase I clinical trial in healthy participants has further validated its favorable safety and pharmacokinetic profile. HRX-215 represents a new therapeutic approach for indications requiring rapid liver repair, offering a new possibility beyond conventional treatments.
About HepaRegeniX GmbH
HepaRegeniX is advancing therapies to treat acute and chronic liver diseases based on the groundbreaking discoveries of a novel cellular target and small molecules that enable the liver to regenerate rapidly. They do so by harnessing the liver’s inherent regenerative power not only in healthy but also in diseased livers. The company’s lead candidate, HRX-215, an orally available small molecule currently in a Phase Ib/IIa trial, selectively inhibits Mitogen-Activated Protein (MAP) Kinase Kinase 4 (MKK4), a master regulator of liver regeneration. Building on demonstrated safety in clinical trials, HepaRegeniX is progressing HRX-215 to prevent post-hepatectomy liver failure, facilitate transplantation of smaller living donor liver grafts, and treat severe alcohol associated hepatitis. Beyond liver diseases, the company is also developing HRX-233 to target kinase inhibitor treatment resistance in KRAS-driven tumors.
HepaRegeniX is backed by experienced life science investors, including Vesalius Biocapital IV, Novo Holdings A/S, Boehringer Ingelheim Venture Fund (BIVF), Coparion, High-Tech Gründerfonds, Ascenion GmbH and Wellington Partners.
Visit our website at www.heparegenix.com to learn more about the company.
For further information, please contact:
HepaRegeniX GmbH
Elias Papatheodorou
Chief Executive Officer
Media Inquiries
Trophic Communications
Charlotte Spitz or Jacob Verghese
Tel: +49 171 351 2733
Email: