ONWARD Medical’s Parkinson’s Therapy Published in New England Journal of Medicine
ONWARD Medical’s Parkinson’s Therapy Published in New England Journal of Medicine
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—April 7, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury, today announces an important publication in the New England Journal of Medicine (NEJM), highlighting the use of ONWARD’s innovative approach to treating orthostatic hypotension in a patient with MSA-P, a degenerative nervous system disease.
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—April 7, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury, today announces an important publication in the New England Journal of Medicine (NEJM), highlighting the use of ONWARD’s innovative approach to treating orthostatic hypotension in a patient with MSA-P, a degenerative nervous system disease.
eGenesis Appoints Eliezer Katz, M.D., FACS, as Chief Medical Officer
eGenesis Appoints Eliezer Katz, M.D., FACS, as Chief Medical Officer
eGenesis Appoints Eliezer Katz, M.D., FACS, as Chief Medical Officer
CAMBRIDGE, Mass., April 04, 2022 – eGenesis, a gene-editing and genome-engineering company developing human-compatible organs and cells, today announced the appointment of Eliezer Katz, M.D., FACS, as Chief Medical Officer (CMO).
“We believe Dr. Katz is an ideal fit for eGenesis because of both his clinical experience as a transplant surgeon and his successful career in the pharmaceutical industry, leading the development of therapeutics in transplant and immunology,” said Paul Sekhri, President and Chief Executive Officer of eGenesis. “His knowledge and expertise have the potential to help us accelerate our progress into clinical trials for our human-compatible organs and cells.”
Dr. Katz is an experienced transplant surgeon and a leader of clinical development in the pharmaceutical industry. He oversaw the development of therapeutics to modulate the immune system in autoimmune diseases and organ transplantation. Most recently, he was Vice President, Clinical Development at Horizon Therapeutics following its acquisition of Viela Bio, Inc. At Horizon and Viela, Dr. Katz led the clinical development of inebilizumab, including the regulatory submission and approval of UPLIZNA® (inebilizumab) to treat neuromyelitis optica spectrum disorder in adults.
Prior to his tenure at Viela and Horizon, Dr. Katz served as Senior Director of Clinical Development at MedImmune Inc. where he oversaw the clinical development of three different biologics. Before that, Dr. Katz served as the Senior Director of Transplantation with the Medicine Development Group at Pfizer, Inc. At Pfizer, he worked across business divisions to oversee multiple research programs with rapamycin, an anti-rejection drug, strengthening his relationships with the medical and surgical transplant community, and conducting key advisory boards. Dr. Katz participated in the planning of Phase 3 clinical trials for tofacitinib, a JAK3 inhibitor and a novel immunosuppressive drug. He was instrumental in the regulatory submission and FDA approval in 2015 of rapamycin as a treatment for lymphangioleiomyomatosis (LAM), a rare and fatal lung disease.
Dr. Katz’s first position in the pharmaceutical industry was at CTI Clinical Trial and Consulting Services where he served as the VP of Medical Affairs and supported all stages of drug development and clinical trials in transplantation, immunology, and other related areas.
Before joining industry, Dr. Katz had an almost two-decade career as a transplant surgeon, including the directorship of two transplantation programs. He was director of the abdominal transplantation division at Integris Baptist Medical Center in Oklahoma City, and an associate professor of surgery and the director of the liver transplantation division at the University of Massachusetts Medical Center, Worcester, MA. In both positions Dr. Katz implemented techniques related to liver transplantation and was actively involved with policy making in organ donation and allocation.
Dr. Katz earned his M.D. at Hadassah Hebrew University Medical School in Jerusalem. “As a transplant surgeon and drug developer, I have seen the huge impact that organ transplantation can have on patients’ lives,” said Dr. Katz. “However, I have also seen the despair of patients who would benefit from a transplant but are unable to access the life-saving surgery due to the significant shortage of available organs. eGenesis’ human compatible organ and cell platform has the potential to address this shortage and radically improve how we treat organ failure. I look forward to working with the eGenesis team to make this mission a reality.”
About Transplantation and Xenotransplantation
The demand for lifesaving organs far outnumbers available supply. In the U.S. alone, more than 100,000 people are on the national transplant list. Twenty people die every day due to lack of available organs for transplant and every 10 minutes, a new name is added to the national transplant waitlist.
The concept of xenotransplantation (the transplantation of organs and cells from one species to another) has been explored for decades, with the pig considered the most suitable donor for humans. However, virology and immunology hurdles prevented the field from advancing beyond early preclinical research. With the advent of cutting-edge gene editing technologies, addressing these historical challenges is now within reach.
About eGenesis
eGenesis’ goal is to transform the field of transplantation by offering safe and effective organs and cells to patients in need. The company harnesses gene editing technology including CRISPR to address the key issues that have impeded xenotransplantation to date. eGenesis’ development pipeline includes lead programs for kidney and islet cell transplant as well as earlier-stage programs focused on other solid organs. Learn more at egenesisbio.com.
Contacts:
Investors/Media
David Carmel
eGenesis, Inc.
212-213-0006
Investors
Eric Ando / Dr. Grace Kim
Burns McClellan, Inc.
212-213-0006
/
Media
Robert Flamm, Ph.D. / Katie Larch
Burns McClellan, Inc.
212-213-0006 ext. 364
/
ONWARD adds new brain-spine interface and Parkinson’s disease IP to license agreements
ONWARD Adds New Brain-Spine Interface and Parkinson’s Disease IP to License Agreements
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—March 28, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury (SCI), today announces two new innovations have been added to its portfolio of license agreements with the Swiss Federal Institute of Technology Lausanne (EPFL), one of the world’s preeminent neuroscience research institutions, and Lausanne University Hospital (CHUV), ranked among the top 10 hospitals in the world.
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—March 28, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury (SCI), today announces two new innovations have been added to its portfolio of license agreements with the Swiss Federal Institute of Technology Lausanne (EPFL), one of the world’s preeminent neuroscience research institutions, and Lausanne University Hospital (CHUV), ranked among the top 10 hospitals in the world.
Adrenomed Supervisory Board appoints Dr. Richard Jones as CEO
Adrenomed Supervisory Board appoints Dr. Richard Jones as CEO
Adrenomed Supervisory Board appoints Dr. Richard Jones as CEO
Hennigsdorf/Berlin (Germany), 15 March, 2022 – Adrenomed AG, the vascular integrity company, today announced the appointment of Dr. Richard Jones as Chief Executive Officer of Adrenomed, effective today. Dr. Jones brings over 25 years of experience in leadership positions in the pharmaceutical and biotech industry with a sustained record of achievements in business, clinical development and commercialization.
“I am delighted to welcome Dr. Richard Jones as the new CEO of Adrenomed. He is a highly achieved life science specialist, who provides a unique combination of strategic, financial, development and organizational capabilities to the Company. Under his leadership, Adrenomed has a strong team in place to lead its first-in-class drug candidate Adrecizumab through late-stage clinical development towards commercialization. Our goal is to bring an urgently needed, effective precision medicine for treating sepsis to intensive care units,” said Prof. Dr. Erich Schlick, Chairman of Adrenomed’s Supervisory Board.
“On behalf of the entire Supervisory Board, I would also like to thank Dr. Wolfgang Baiker, former CEO of Adrenomed, for his contribution to the company and wish him well for the future,” he added.
Dr. Richard Jones, CEO of Adrenomed, said: “I am excited to be joining Adrenomed at a pivotal phase of the Company’s evolution. Sepsis is responsible for between one third and one half of deaths of hospitalized people. This equates to 11 million people worldwide dying each year which is higher than the top 3 cancer conditions combined and represents a significant high unmet medical need. Adrenomed’s innovative biomarker guided treatment approach, with Adrecizumab, is targeting the endothelial barrier dysfunction as a major cause of mortality in sepsis. I look forward to working with the team and advancing the late-stage clinical development of Adrecizumab to provide a new treatment option for critically ill patients.”
Richard Jones joins Adrenomed from Fusion Antibodies Plc, a contract research organization specialized in antibody engineering. As CEO of Fusion Antibodies Plc, Richard successfully implemented a new growth strategy in the global antibodies marketplace. Previously, Richard served as CEO for a number of UK and European private companies as well as SVP, Head of Europe for a US based public company. Before this he gained expertise at Novartis and GSK as VP, Medicines Commercialization Leader Global Haematology at both companies. In these roles, Richard was responsible for three regulatory submissions and initiation of several new drug development programs, plus the planning and execution of three global product launches. Richard also worked with Genzyme Corporation and as International Franchise Director for Shire Pharmaceuticals. He holds a BSc in Biochemistry and PhD in Molecular Oncology from University of Surrey.
About Adrenomed
Adrenomed AG is a German privately financed, clinical-stage biopharmaceutical company. Adrenomed’s mission is to rescue vascular integrity in order to save the lives of critically ill patients with limited treatment options. Founded in 2009 by a management team with decades of in-depth experience in sepsis and deep knowledge in diagnostics and drug development, the company’s lead product candidate Adrecizumab is a first-in-class monoclonal antibody. Adrecizumab targets the vasoprotective peptide Adrenomedullin, an essential regulator of vascular integrity. Adrecizumab has successfully completed a biomarker-guided, double-blinded, placebo-controlled, randomized, multicenter proof-of-concept Phase II trial with 301 patients suffering from septic shock. For further information, please visit www.adrenomed.com and follow us on LinkedIn and Twitter.
Contact
Adrenomed AG
Frauke Hein, Ph.D. (Chief Business Officer)
phone: +49 (0)3302 2077814
Media Inquiries
MC Services AG
Eva Bauer / Julia von Hummel
phone: +49 (0)89 21022880
First participants enrolled in ONWARD’s LIFT home study
First participants enrolled in ONWARD’s LIFT home study
Study will evaluate safety and performance of ARCEX therapy for spinal cord injury in home setting
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—March 9, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury, today announces the start of enrollment in the LIFT Home Study. The trial is designed to study the safety and performance of ARCEX Therapy when used in the home.
ONWARD’s ARCEX Therapy is externally delivered programmed stimulation of the spinal cord to restore strength and function in people with spinal cord injury and other movement related challenges. The study will enroll up to 20 participants at 5 leading research centers in the United States: Craig Hospital in Denver, Colorado, Shepherd Center in Atlanta, Georgia, Spaulding Research Institute in Boston, Massachusetts, University of Minnesota, and University of Washington. Subjects will be followed for four weeks to assess whether continued access to ONWARD’s ARCEX Therapy is safe and can be used to enhance long-term benefit.
The first participants have been enrolled at Craig Hospital under the supervision of study principal investigator, Candy Tefertiller, PT, DPT, Ph.D., NCS, Executive Director of Research and Evaluation at Craig and at the University of Washington in Seattle by Chet Moritz, PhD, Associate Professor in the Departments of Electrical& Computer Engineering and Rehabilitation Medicine. “The LIFT Home Study is an important next step in understanding the potential benefits people with spinal cord injury may derive from continued access to ARC Therapy outside the clinic,” said Dr. Tefertiller.
The LIFT Home Study is a successor to ONWARD’s Up-LIFT Study, a pivotal trial that completed enrollment of 65 participants in December 2021, with participating research centers in the US, Canada, the UK, and Europe. Up-LIFT is designed to demonstrate ONWARD ARCEX Therapy can improve the strength and function of upper limbs when used in the rehabilitation clinic setting.
The LIFT Home Study seeks to evaluate the potential impact of ARC EX Therapy when used in the home setting.
“We are pleased to collaborate with an outstanding group of researchers to investigate new potential benefits and care settings for our ARC EX Therapy,” said Dave Marver, CEO of ONWARD. “This is another step in our journey to help people with spinal cord injury live better, more independent lives.”
To learn more about ONWARD’s ARC Therapy and the company’s vision to restore movement, independence and health in people with spinal cord injury, please visit ONWD.com.
About ONWARD
ONWARD is a medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury. ONWARD’s work builds on more than a decade of basic science and preclinical research conducted at the world’s leading neuroscience laboratories. ONWARD’s ARC Therapy, which can be delivered by implantable (ARC IM) or external (ARCEX ) systems, is designed to deliver targeted, programmed stimulation of the spinal cord to restore movement and other functions in people with spinal cord injury, ultimately improving their quality of life. ONWARD has received three Breakthrough Device Designations from the FDA encompassing both ARCIM and ARCEX . The company’s first FDA pivotal trial, called Up-LIFT, completed enrollment in December 2021 with 65 subjects worldwide.
ONWARD is headquartered at the High Tech Campus in Eindhoven, the Netherlands. It maintains a significant team in Lausanne, Switzerland and has a growing U.S. presence in Boston, Massachusetts, USA. For additional information about the company, please visit ONWD.com. To access our 2022 Financial Calendar, please visit IR.ONWD.com.
For Media Enquiries:
Simon Gentry
+44 (0)20 3757 6772
For Company Enquiries:
ONWARD
For Investor Enquiries:
Disclaimer
Certain statements, beliefs and opinions in this press release are forward-looking, which reflect the Company or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve a number of risks, uncertainties and assumptions that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, changes in demand, competition and technology, can cause actual events, performance or results to differ significantly from any anticipated development. Forward looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.
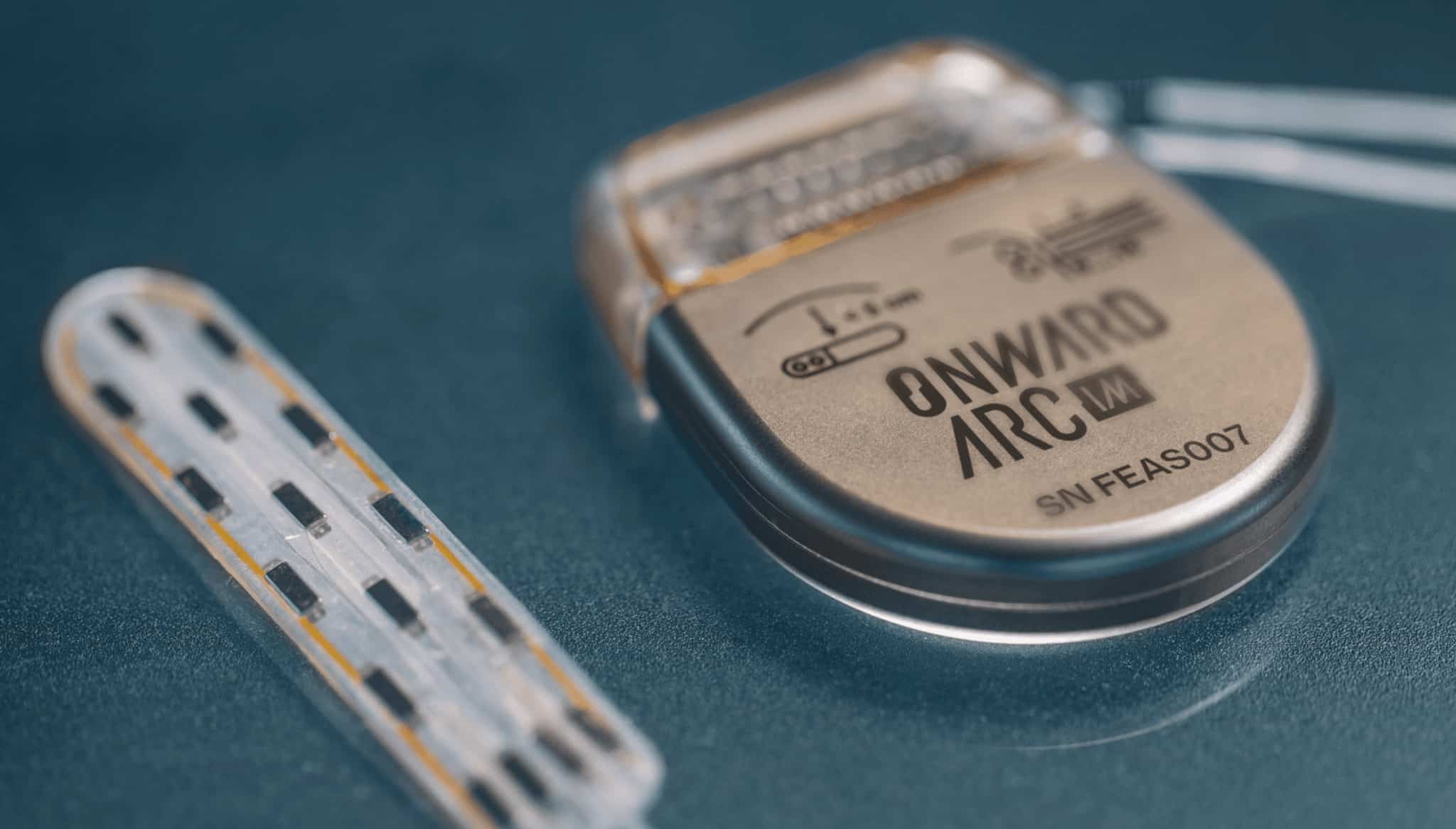
ONWARD medical’s technology enables walking and standing after severe spinal cord injuries
ONWARD medical’s technology enables walking and standing after severe spinal cord injuries
STIMO–BRIDGE study results published in Nature Medicine
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—February 7, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury, today announces the publication of STIMO-BRIDGE Study results in Nature Medicine. The study highlights the use of ONWARD’s technology to enable people with even the most severe forms of spinal cord injury to walk, stand, cycle, and swim again.
The STIMO-BRIDGE study was conducted by NeuroRestore, a collaboration between the Swiss Federal Institute of Technology (EPFL) and the Centre Hospitalier Universitaire Vaudois (CHUV, which is co-led by Professor Grégoire Courtine, PhD and Neurosurgeon Jocelyne Bloch, MD. It was published today in NATURE Medicine, entitled, “Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis.”
The study leveraged an innovative lead developed by ONWARD to specifically target the areas of the spinal cord involved in leg and lower trunk movement with the intent to facilitate faster and more effective recovery of motor function. To design this lead, ONWARD and NeuroRestore used an advanced computational models and spinal cord atlas to determine optimal electrode placement and guide optimal position during surgery. The models for pre-operative planning and for intra- operative neuromonitoring were developed within the framework of RESTORE and CONFIRM Eurostar projects, in collaboration with Utrecht University Medical Imaging Center, the IT’IS Foundation, ZMT Zurich MedTech AG, and Medical Faculty of Heidelberg University.
Three participants with complete sensorimotor spinal cord injury (AIS-A) were implanted with this new lead. Prior to implantation, these subjects could neither contract their leg muscles nor take a single step. On the first day following implant, all participants were able to take steps independently on a treadmill with body weight support.
After 5 months of rehabilitation, participants were able to use their legs to stand, walk, swim, and/or cycle. They also regained control of their trunk muscles. This recovery of leg and trunk motor function also enabled participants to stand independently in community settings. This video from EPFL describes the study and the gains enjoyed by participants.
“Enabled by ONWARD technology, Professor Courtine and colleagues have demonstrated a remarkable breakthrough in restoring the ability to stand and walk even in people with the most severe spinal cord injuries,” said Dave Marver, CEO of ONWARD. “We are working hard to bring these therapies to the SCI community as soon as possible and our first introduction, for restoration of hand and arm function, is expected in early 2023.”
To learn more about ONWARD’s ARC Therapy and the company’s vision to restore movement, independence and health in people with spinal cord injury, please visit ONWD.com.
About ONWARD
ONWARD is a medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury. ONWARD’s work builds on more than a decade of basic science and preclinical research conducted at the world’s leading neuroscience laboratories. ONWARD’s ARC Therapy, which can be delivered by implantable (ARCIM) or external (ARCEX) systems, is designed to deliver targeted, programmed stimulation of the spinal cord to restore movement and other functions in people with spinal cord injury, ultimately improving their quality of life. ONWARD has received three Breakthrough Device Designations from the FDA encompassing both ARCIM and ARCEX. The company’s first FDA pivotal trial, called Up-LIFT, completed enrollment in December 2021 with 65 subjects worldwide. ONWARD’s technology is protected by over 310 issued or pending patents globally.
ONWARD is headquartered at the High Tech Campus in Eindhoven, the Netherlands. It maintains a significant team in Lausanne, Switzerland and has a growing U.S. presence in Boston, Massachusetts, USA. For additional information about the company, please visit ONWD.com. To access our 2022 Financial Calendar, please visit IR.ONWD.com.
For Company Enquiries:
ONWARD
For Media Enquiries:
Simon Gentry
+44 (0)20 3757 6772
For Investor Enquiries:
Backstage Communication
Gunther De Backer
Tel: +32 (0)475 903 909
Disclaimer
Certain statements, beliefs and opinions in this press release are forward-looking, which reflect the Company or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve a number of risks, uncertainties and assumptions that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, changes in demand, competition and technology, can cause actual events, performance or results to differ significantly from any anticipated development. Forward looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.
Moderna and Carisma establish collaboration to develop in vivo engineered Chimeric Antigen Receptor Monocytes (CAR-M) for Oncology
Moderna and Carisma establish collaboration to develop in vivo engineered Chimeric Antigen Receptor Monocytes (CAR-M) for Oncology
- Collaboration will combine Carisma’s engineered macrophage technology with Moderna’s mRNA and LNP technologies to generate and develop in vivo CAR-M therapeutics
- Multi-year research collaboration funded by Moderna with options for up to twelve targets
- Carisma to receive $45 million up-front cash payment and investment by Moderna in the form of a $35 million convertible note
- Carisma eligible to receive milestone and royalty payments
CAMBRIDGE, MA, and PHILADELPHIA, PA – January 10, 2022 – Moderna Inc. (NASDAQ:MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, and Carisma Therapeutics Inc., a biopharmaceutical pioneer in engineered macrophage-based therapeutics, today announced that the two companies have entered into a strategic collaboration agreement to discover, develop and commercialize in vivo engineered chimeric antigen receptor monocyte (CAR-M) therapeutics for the treatment of cancer.
“We are excited to begin this collaboration with Carisma to further expand our oncology pipeline with a differentiated in vivo cell-therapy approach,” said Stephen Hoge, President of Moderna. “This exemplifies our strategy to partner with companies with deep biological expertise while leveraging Moderna’s core mRNA and LNP capabilities to further expand the reach of Moderna’s technology.”
“Moderna’s deep expertise in mRNA and LNP technologies opens up a potentially game-changing opportunity for engineered macrophages,” said Steven Kelly, President and Chief Executive Officer of Carisma. “In vivo delivery directly to monocytes and macrophages enables an off-the-shelf therapeutic approach that uses the patients’ own cells to provide a truly personalized treatment. By combining Carisma’s expertise in engineered macrophage biology and Moderna’s pioneering in vivo mRNA delivery technologies, we are excited about the potential of this novel therapeutic approach for treating cancer. We are thrilled to be working with Moderna.”
About the Collaboration
Under the terms of the agreement, Carisma will receive a $45 million up-front cash payment and an investment by Moderna in the form of a $35 million convertible note. Carisma will receive research funding and is eligible to receive development, regulatory, and commercial milestone payments, plus royalties on net sales of any products that are commercialized under the agreement. Carisma will be responsible for the discovery and optimization of development candidates while Moderna will lead the clinical development and commercialization of therapeutics resulting from the agreement. Moderna has the option to nominate up to twelve targets for development and commercialization.
About Moderna
In 10 years since its inception, Moderna has transformed from a research-stage company advancing programs in the field of messenger RNA (mRNA), to an enterprise with a diverse clinical portfolio of vaccines and therapeutics across six modalities, a broad intellectual property portfolio in areas including mRNA and lipid nanoparticle formulation, and an integrated manufacturing plant that allows for both clinical and commercial production at scale and at unprecedented speed. Moderna maintains alliances with a broad range of domestic and overseas government and commercial collaborators, which has allowed for the pursuit of both groundbreaking science and rapid scaling of manufacturing. Most recently, Moderna’s capabilities have come together to allow the authorized use of one of the earliest and most-effective vaccines against the COVID-19 pandemic.
Moderna’s mRNA platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, and has allowed the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, cardiovascular diseases and auto-immune diseases. Moderna has been named a top biopharmaceutical employer by Science for the past seven years. To learn more, visit www.modernatx.com.
About Carisma Therapeutics
Carisma is a biopharmaceutical company dedicated to developing a differentiated and proprietary cell therapy platform focused on engineered macrophages, cells that play a crucial role in both the innate and adaptive immune response. The first applications of the platform, developed in collaboration with the University of Pennsylvania, are autologous chimeric antigen receptor (CAR)-macrophages for the treatment of solid tumors. Carisma is headquartered in Philadelphia, PA.
For more information, please visit www.carismatx.com
Moderna Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including regarding: Moderna’s collaboration with Carisma to discover, develop and commercialize CAR-M therapeutics for the treatment of cancer; the terms of that collaboration; and the potential for the collaboration to lead to personalized cancer treatments. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties, and other factors include those risks and uncertainties described under the heading “Risk Factors” in Moderna’s most recent Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (SEC) and in subsequent filings made by Moderna with the SEC, which are available on the SEC’s website at www.sec.gov. Except as required by law, Moderna disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on Moderna’s current expectations and speak only as of the date hereof.
Moderna Contacts:
Media:
Colleen Hussey
Director, Corporate Communications
617-335-1374
Investors:
Investors:
Lavina Talukdar
Senior Vice President & Head of Investor Relations
617-209-5834
Carisma Media Contact:
Creative Balloons appoints David Johnson as Chairman of the Board of Directors
Creative Balloons appoints David Johnson as Chairman of the Board of Directors
Creative Balloons GmbH, Waghäusel near Heidelberg, a specialist in medical technology, today announced the appointment of David Johnson as Chairman of the Board of Directors, effective January 1, 2022.
Creative Balloons appoints David Johnson as Chairman of the Board of Directors
|
DGAP-News: Creative Balloons GmbH / Key word(s): Personnel Creative Balloons appoints David Johnson as Chairman of the Board of Directors
Waghaeusel (Germany), January 10, 2022 – Creative Balloons GmbH, Waghäusel near Heidelberg, a specialist in medical technology, today announced the appointment of David Johnson as Chairman of the Board of Directors, effective January 1, 2022. Dave has held many senior executive and board positions with large market-leading companies as well as emerging companies with innovative medical devices. Managing global medtech businesses for over 20 years in Canada, the USA and the UK, he has successfully developed mid-, small- and micro-cap companies in public and private environments. “We are delighted to welcome David Johnson as Chairman of the Creative Balloons’ Board of Directors,” said Frank Gehres, CEO of Creative Balloons. “His many years of executive and board experience with large and smaller players in the medtech industry will be a tremendous asset for Creative Balloons as we approach market entry into the US and UK. Dave’s strategic advice and strong industry expertise, specifically in the intensive care segment, as well as his deep insight into the US market will help us to accomplish important tasks such as building up our commercial organization and achieving market access for our product hygh-tec(R). I look forward to working closely with him and our Board as we carry out our mission to rethink critical care.” David Johnson has 35 years of experience leading small, medium, and large companies – both public and private – in the medical device space, including intensive care products. Before heading the Creative Balloons board, Dave served as CEO for Enveric Biosciences, and continues as a member of their Board of Directors. “I strongly believe in the highly innovative approach to catheters that Creative Balloons has developed with hygh-tec(R),” commented David Johnson. “Its successful market entry in Germany is impressive. I am very much looking forward to working with Creative Balloons as it grows into new markets and to bringing in my business experience from that specific product category. It will be a pleasure to help leverage the full potential of the contribution hygh-tec(R) brings to ICU caregivers and the enhanced treatment opportunities it holds for patients.” “The Board of Creative Balloons is excited about Dave Johnson joining and leading our efforts,” says Dr. Ulrich Wandschneider, former CEO of hospital operator Asklepios and current member of the board of directors of BioNTech, who is representing investment company Salvia on the Creative Balloons Board of Directors. Besides him and David Johnson, the Board consists of Dr. Karl Nägler representing Wellington Partners and Matthias Guth representing MIG Capital AG. About Creative Balloons Contact: Media relations: 10.01.2022 Dissemination of a Corporate News, transmitted by DGAP – a service of EQS Group AG. The DGAP Distribution Services include Regulatory Announcements, Financial/Corporate News and Press Releases. |