MMI Raises $110 Million in Series C Financing
MMI Raises $110 Million in Series C Financing
Largest ever investment in microsurgery will further MMI’s mission to transform open surgery with robotic technology
JACKSONVILLE, Fla.–(BUSINESS WIRE)–MMI (Medical Microinstruments, Inc.), a robotics company dedicated to increasing treatment options and improving clinical outcomes for patients with complex conditions, today announced that it has raised $110 million in Series C financing. The round, led by Fidelity Management & Research Company, marks the largest ever investment in microsurgery innovation.
The funds will support commercialization of the Symani® Surgical System in high-growth markets and continued investment in studies that generate clinical evidence and enable indication expansion. Investments will also accelerate advanced technology capabilities and enable MMI to scale its operational capabilities globally.
MMI and its existing investors, all of whom contributed to the Series C financing, see considerable opportunity for rapid growth. The company projects the market for eligible robotic microsurgical procedures will grow from 3 million to 22 million annually by 2028, driven primarily by technological advancements and indication expansion.
“Against a backdrop of plateauing investments in medical robotics, this support builds on our confidence in a new, less invasive solution for open surgery, a significant market that can benefit from the smallest wristed microinstruments,” said Mark Toland, CEO of MMI. “Our Symani Surgical System is uniquely positioned to expand patient access to care by accelerating the number of surgeons able to perform complex, delicate procedures. With the support of our investors, we will continue to advance our technology through a growing body of clinical evidence and expanded hospital partnerships.”
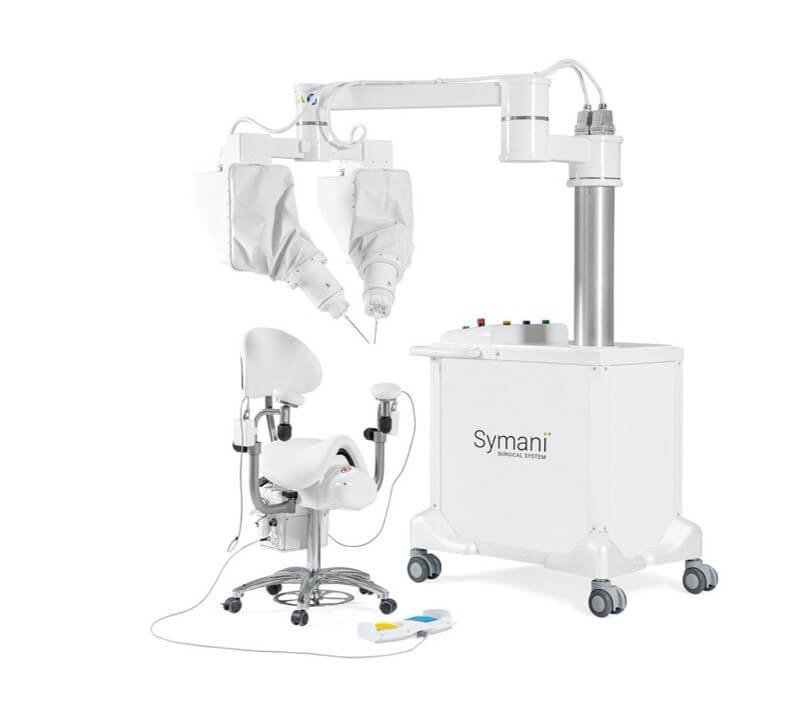
The Symani Surgical System is a first-of-its-kind robotic technology that uniquely addresses the scale and complexities of microsurgery and supermicrosurgery. By allowing surgeons to replicate the natural movements of the human hand at the micro scale, it can expand treatment options for patients in need of soft tissue, open surgical procedures, such as free flap reconstructions, lymphatic surgery, and trauma replantations. It is designed to help restore quality of life for more patients, accelerate the number of surgeons able to push the boundaries of complex procedures for delicate anatomy, and enable hospitals to expand their open surgical programs.
MMI has raised over $200 million in funding to date. In 2022, it closed a Series B financing round to propel growth. The funding was allocated to help expand indications and support ongoing commercialization efforts for the Symani Surgical System in Europe, where it received CE mark in 2019, accelerate plans to commercialize in the U.S. and Asia-Pacific, and advance clinical research.
To learn more about MMI and the Symani Surgical System, visit MMI’s website here: https://mmimicro.com.
About MMI
MMI (Medical Microinstruments, Inc.) is on a mission to advance robotic technology that pushes the limits of soft tissue open surgery and opens new opportunities for surgeons to restore quality of life for more patients with complex conditions. The company was founded in 2015 near Pisa, Italy, and its proprietary Symani® Surgical System combines the world’s smallest wristed microinstruments with tremor-reducing and motion-scaling technologies to address significant unmet patient needs across the globe. This first-of-its-kind surgical robotic platform for open, soft tissue micro-level surgery can help address microvascular repair, lymphatic repair, and peripheral nerve repair. The Symani System is CE Marked for commercial use in Europe. In the United States, the system is not approved or cleared for commercial use. MMI is backed by global investors including Fidelity Management & Research Company, Andera Partners, BioStar, Deerfield Management, Fountain Healthcare Partners, Panakès Partners, RA Capital, Sambatech, and Wellington Partners.

UroMems Announces Results of First-Ever Smart Artificial Urinary Sphincter Implant in Female Patient to Treat Stress Urinary Incontinence
UroMems Announces Results of First-Ever Smart Artificial Urinary Sphincter Implant in Female Patient to Treat Stress Urinary Incontinence
Positive results demonstrate promising new option for women seeking better, personalized treatment
GRENOBLE, France and MINNEAPOLIS, Feb. 14, 2024 /PRNewswire/ — UroMems, a global company developing innovative, mechatronics technology to treat stress urinary incontinence (SUI), announced today that it has successfully met the six-month primary endpoint for the first-ever female patient implanted with the UroActive™ System, the first smart automated artificial urinary sphincter (AUS) to treat SUI. This milestone indicates a new era for millions of women suffering from SUI, and signals an exciting transition for surgeons treating SUI not only in France, where the female patient was treated, but also across Europe and the U.S. It also follows closely on the heels of the successful results of the complete treatment cohort of the first-in-man clinical feasibility study. Results of this clinical study will contribute to the design and implementation of UroMems’ pivotal clinical trial in Europe and the U.S.
“On behalf of the medical team who implanted the UroActive System in the first female patient, Drs. Christophe Vaessen, Aurelien Beaugerie and I are over the moon to see our first patient has returned to living life fully after years of struggling with SUI,” said Professor Emmanuel Chartier-Kastler. “This promising therapy is a breakthrough technology in treating SUI in both women and men.”
The primary outcome measures include the successful device activation and the rate of explants and revisions at six months. The first female patient has not only met the study’s primary endpoints by remaining revision-free but is also experiencing restored social continence. Follow-up on secondary measures, including leak rate values, has been extremely positive.
“As the leading advocacy organization in the U.S. supporting patients and caregivers with urinary incontinence, we hear from patients weekly looking for information on the safety and efficacy of artificial sphincters. Unfortunately, we have little to report back and support their requests as no one in the U.S. actively promotes this option for women,” said Steven Gregg, Ph.D., executive director of the National Association for Continence. “These results of the first UroActive System implanted in a female represent a promising development to treat stress urinary incontinence in both women and men. We’re excited about this first-of-its-kind development and look forward to UroMems’ pivotal trial results.”
Pending those results, the potential for U.S. surgeons to offer this new option is now on the horizon; skilled surgeons performing robotic-assisted surgeries such as sacrocolpopexies and hysterectomies may soon be able to add implanting UroActive to their standard practices. UroActive is the first smart active implant that treats SUI, powered by a MyoElectroMechanical System (MEMS). This innovative system is placed around the urethra in men and the bladder neck in women, controlled based on the patient’s activity, without the need for manual adjustments, intending to provide patients with ease of use and a better quality of life than current options.
“We are elated to reach this critical achievement contributing to the demonstration of the feasibility of the UroActive System to successfully treat women suffering from debilitating SUI,” said Hamid Lamraoui, UroMems chief executive officer and co-founder. “The compelling results of this first-in-female implant show the high potential of our technology, bringing us one step closer to delivering on the massive unmet need for women and physicians desperately seeking a better SUI treatment option.”
SUI, or involuntary urinary leakage, affects an estimated 40 million Americans and 90 million Europeans, and occurs when the pressure in the bladder exceeds that of the muscle (the sphincter) around the urethra, caused by activities involving high intra-abdominal pressure, like coughing, laughing and exercising. SUI significantly impacts quality of life, as it can be debilitating, and often leads to depression, low self-esteem and social stigma.
UroMems aims to restore the quality of life, dignity and self-esteem of millions of men and women worldwide suffering from poorly treated chronic conditions by the commitment to change the perception that these disorders are inevitable as one grows older and is simply something to endure with no real solution. UroMems is revolutionizing the treatment of SUI with smart active implants, using the latest technological advances in the field of embedded systems and micro-technologies for the development of its groundbreaking solutions.
About UroActive
UroActive is an active implantable electronic artificial urinary sphincter that is being developed to compensate for sphincter insufficiency in patients, both men and women, with SUI. It is based on a unique bionic platform using embedded smart, digital and robotic systems which, based on data collected from a patient, create a treatment algorithm that is specific for each patient’s needs. The UroMems technology platform is protected by more than 120 patents and is designed to overcome the limitations of current solutions by optimizing safety and performance, patient experience and surgeon convenience. STeP participation does not imply product authorization. UroActive has not received marketing authorization from the FDA and is not available for sale in the United States or in the EU.
For more information, please visit www.uromems.com.
Media Contact:
Shelli Lissick
651-276-6922

eGenesis and OrganOx Announce Successful Use of a Genetically Engineered Porcine Liver with a Human Donor
eGenesis and OrganOx Announce Successful Use of a Genetically Engineered Porcine Liver with a Human Donor
72-hour proof-of-concept procedure at Penn Medicine marks longest perfusion using genetically
engineered porcine organ designed for liver support
Cambridge, Mass. (January 18, 2023) – eGenesis, a biotechnology company developing human-compatible organs and cells for the treatment of organ failure, and OrganOx, a medical device company with a focus on the therapeutic applications of isolated organ perfusion, today announced the successful completion of an extracorporeal perfusion of a brain-dead research donor using a genetically engineered porcine liver. The donor was the first to be enrolled in the ongoing PERFUSE-2 study.
The study, made possible through the generosity of a donor family seeking to help other families through the advancement of clinical research, was carried out in collaboration with the University of Pennsylvania Transplant Institute and Gift of Life Donor Program. The perfusion was performed using the eGenesis liver, EGEN-5784, connected to the OrganOx extracorporeal liver cross-circulation (ELC) device to enable circulation of the donor’s blood through the porcine liver. Stable blood flow, pressure, and pH were achieved throughout the procedure in addition to robust bile production. No evidence of rejection was observed. The perfusion was electively stopped per-protocol at 72 hours with the liver appearing healthy.
The PERFUSE-2 study is being conducted to evaluate the feasibility of using this liver perfusion system to support patients suffering from liver failure. Annually in the US, over 300,000 patients are admitted with various forms of liver failure, requiring treatment in the acute setting. The efficacy of existing liver support options is limited, and patients in liver failure face a high risk of mortality. For some patients, utilizing a human-compatible, genetically engineered porcine whole liver to support the function of a patient’s decompensated liver may provide time for the recovery of the patient’s native liver or time to obtain a liver transplant. eGenesis and OrganOx are co-developing this technology and anticipate submitting an Investigational New Drug (IND) Application to the U.S. Food and Drug Administration (FDA) in 2024 to initiate a first-in-human clinical study.
The genetically engineered porcine liver used in this study carried the same genetics as the porcine kidneys used in the landmark preclinical study recently published in Nature. These edits include (1) knock out of three genes involved in the synthesis of glycan antigens implicated in hyperacute rejection, (2) insertion of seven human transgenes involved in the regulation of several pathways that modulate rejection: inflammation, innate immunity, coagulation, and complement, and (3) inactivation of the endogenous retroviruses in the porcine genome.
“We wish to extend our deepest gratitude to the donor and their family for enabling this important medical achievement and for paving the way for future work toward a potential solution for the many patients in need of life-saving liver support,” said Michael Curtis, Ph.D., President and Chief Executive Officer of eGenesis. “In addition to being a first in the field of xenotransplantation, this study provides important information to help advance our IND application.”
Abraham Shaked, M.D., Ph.D. of the University of Pennsylvania Transplant Institute said, “The success of this study provides support for further exploration of organ products developed using advanced genome engineering to provide novel, high-quality therapeutic options for individuals experiencing organ failure.”
“This marks a key milestone in our journey towards an effective treatment for acute liver decompensation. The OrganOx ELC system combined with a genetically engineered liver from eGenesis links modern organ perfusion technology with the functions of a whole liver, with the goal of providing a treatment that offers a lifeline to the critically ill patient – time for their own liver to recover or time to receive a transplant,” said Prof Peter Friend, Chief Medical Officer, OrganOx.
“Gift of Life Donor Program, one of the nation’s leading organ procurement organizations, is proud to have collaborated on this pioneering study that has the potential to bring new hope to waitlist patients by providing a bridge to transplant or time for healing,” said Richard D. Hasz, Jr., MFS, CPTC, President and CEO, Gift of Life Donor Program. “This unique study was only possible thanks to the generosity of a donor family willing to help alleviate the suffering of others despite their own personal loss. Every day, we are inspired by the kindness of families who choose donation. We look forward to partnering with Penn Medicine, eGenesis and OrganOx on future trials to advance this evolving field of medicine.”
“Our family is very proud to support this medical advancement and see our loved one’s legacy benefit countless others,” said a member of the donor family. “It is a testament to our loved one’s selflessness and compassion to know this donation offers such hope for people suffering serious disease in the future.”
About OrganOx
OrganOx is a commercial stage UK-based medical device company with a focus on the therapeutic applications of isolated organ perfusion. Its first product, the OrganOx metra normothermic liver machine perfusion system, has been used to support more than 3000 liver transplant operations globally, optimizing the use of donated organs by enabling assessment of the quality of livers as well as longer preservation durations. Other therapeutic applications, including in kidney transplantation, are in development. Learn more at www.organox.com
About eGenesis
eGenesis is pioneering a genome engineering-based approach in the development of safe and effective transplantable organs. The eGenesis Genome Engineering and Production (EGEN™) Platform is the only technology of its kind to comprehensively address cross-species molecular incompatibilities and viral risk via genetic engineering. eGenesis has demonstrated durable preclinical success to date and is advancing development programs for acute liver failure, kidney transplant, and pediatric as well as adult heart transplant. Learn more at www.egenesisbio.com.
Media Contact
eGenesis Kimberly Ha
OrganOx
Daniel Woodward
First In Vivo CAR-M Lead Candidate Nominated Under Carisma-Moderna Collaboration
First In Vivo CAR-M Lead Candidate Nominated Under Carisma-Moderna Collaboration
First lead candidate to address a solid tumor indication with significant unmet medical need
Supportive pre-clinical proof of concept data reported at SITC 2023 that demonstrated feasibility, tolerability and early efficacy of in vivo CAR-M therapy utilizing mRNA/LNPs in solid tumors
PHILADELPHIA, Dec. 14, 2023 /PRNewswire/ — Carisma Therapeutics Inc. (Nasdaq: CARM) (“Carisma” or the “Company”), a clinical-stage biopharmaceutical company focused on discovering and developing innovative immunotherapies, today announced the nomination of its first lead candidate under the collaboration with Moderna, Inc. (Nasdaq: MRNA). This first lead candidate will target an antigen present on a solid tumor with significant unmet medical need. This strategic collaboration brings together Carisma’s chimeric antigen receptor macrophage (CAR-M) platform with Moderna’s messenger RNA (mRNA) and lipid nanoparticle (LNP) technologies to generate and develop in vivo CAR-M therapeutics for oncology.
“Following the compelling pre-clinical proof of concept data shared at SITC 2023, we believe in vivo CAR-M therapeutics that utilize Moderna’s mRNA/LNPs have the potential to benefit patients with a broad variety of cancers,” stated Michael Klichinsky, PharmD, PhD, Co-Founder and Chief Scientific Officer at Carisma. “The delivery of this first candidate demonstrates our ability to create novel in vivo CAR therapies that can be advanced toward the clinic. We are proud of the significant contributions made by both of the scientific teams at Carisma and Moderna towards this exciting program. We look forward to completing IND-enabling studies with the lead candidate and are excited about the prospect of bringing this therapy forward for patients with advanced solid tumors together with Moderna.”
Lin Guey, PhD, Chief Scientific Officer of Therapeutics Research Ventures and Biotherapeutics at Moderna, stated, “We are excited with the progress we’ve made to advance in vivo cell therapy (CAR-M) in collaboration with Carisma. Combining Carisma’s deep expertise in myeloid cell biology with our mRNA/LNP platform has allowed us to quickly advance the first lead candidate and we look forward to furthering its development, along with our continued collaboration with Carisma to develop novel therapies to treat patients.”
Pre-clinical proof of concept data were recently presented at SITC 2023, demonstrating the feasibility, efficacy, and tolerability of the in vivo CAR-M platform that utilizes Moderna’s optimized mRNA encapsulated in LNPs and is designed to redirect endogenous myeloid cells to exert targeted anti-tumor activity. The highlighted data demonstrated that Carisma’s CAR-M therapy can be directly produced in vivo, or within the body, and can successfully redirect endogenous myeloid cells against tumor-associated antigens using Moderna’ mRNA/LNPs. This novel approach to cancer immunotherapy offers an off-the-shelf solution that has the potential to increase access to CAR-based therapies and to be the basis of any CAR-M programs discovered or developed under the Carisma and Moderna collaboration.
About Carisma
Carisma Therapeutics Inc. is a clinical stage biopharmaceutical company focused on utilizing our proprietary macrophage and monocyte cell engineering platform to develop transformative immunotherapies to treat cancer and other serious diseases. We have created a comprehensive, differentiated proprietary cell therapy platform focused on engineered macrophages and monocytes, cells that play a crucial role in both the innate and adaptive immune response. Carisma is headquartered in Philadelphia, PA. For more information, please visit www.carismatx.com.
Cautionary Note on Forward-Looking Statements
Statements in this press release about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, statements relating to Carisma’s business, strategy, future operations, cash runway, the advancement of Carisma’s product candidates and product pipeline, and pre-clinical and clinical development of Carisma’s product candidates, including expectations regarding timing of initiation and results of pre-clinical and clinical trials. The words “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “goals,” “intend,” “may,” “might,” “outlook,” “plan,” “project,” “potential,” “predict,” “target,” “possible,” “will,” “would,” “could,” “should,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. For a discussion of these risks and uncertainties, and other important factors, any of which could cause Carisma’s actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” set forth in the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 9, 2023, as well as discussions of potential risks, uncertainties, and other important factors in Carisma’s other recent filings with the Securities and Exchange Commission. Any forward-looking statements that are made in this press release speak as of the date of this press release. Carisma undertakes no obligation to revise the forward-looking statements or to update them to reflect events or circumstances occurring after the date of this press release, whether as a result of new information, future developments or otherwise, except as required by the federal securities laws.
Contacts
Investors:
Shveta Dighe
Head of Investor Relations
Media:
Julia Stern
(763) 350-5223
SOURCE Carisma Therapeutics Inc.
UroMems Reaches Significant Milestone: Successful Results in Clinical Feasibility Study of UroActive™ Smart Implant for Stress Urinary Incontinence Treatment
UroMems Reaches Significant Milestone: Successful Results in Clinical Feasibility Study of UroActive™ Smart Implant for Stress Urinary Incontinence Treatment
Successful primary and secondary results demonstrate proof of feasibility in male patients, paving way for launch of large-scale pivotal clinical study in the U.S. and Europe
GRENOBLE, France and MINNEAPOLIS, Dec. 13, 2023 /PRNewswire/ — UroMems, a global company developing innovative, mechatronics technology to treat stress urinary incontinence (SUI), announced today it has reached a significant milestone: the complete treatment cohort in the first-of-its-kind clinical feasibility study has successfully reached the six-month primary endpoints.
The feasibility assessment of the UroActive System was completed through a prospective multicenter clinical study. UroActive is the first smart automated artificial urinary sphincter (AUS) to treat SUI, and the only one to reach this critical milestone. The results of this initial clinical study support design and implementation of UroMems’ pivotal SUI trial in Europe and the U.S. All six men are now implanted for at least seven months and up to fifteen months, with their devices operating as expected and no need for revision nor explant. In addition, extremely positive follow-up was received on secondary outcomes measures, including leak rate values and patient quality of life questionnaires.
UroMems received very positive feedback from all patients participating in the study cohort. “You have changed my life,” stated one of the study participants. “I can do all activities again, without stress, without anxiety!”
“We’re so pleased to see that our expectations about our device’s performance were met or even exceeded and delighted to successfully treat and receive such high praise from patients who had been suffering from SUI for years,” said Professor Pierre Mozer, UroMems chief medical officer and co-founder. “The results of this study will allow us to prepare our pivotal study which will be a major step in the development of UroActive.”
UroActive is the first smart active implant that treats SUI, powered by a MyoElectroMechanical System (MEMS). This innovative system is placed around the urethral duct and is controlled based on the patient’s activity, without the need for manual adjustments, intending to provide patients with ease of use and a better quality of life than current options.
“The very compelling results of this first-in-man clinical study demonstrate the high potential of our technology and pave the way for larger clinical trials that will allow us to demonstrate all the benefits we are expecting to offer patients suffering from debilitating SUI,” said Hamid Lamraoui, UroMems chief executive officer and co-founder. “We could not have reached this important milestone without the enthusiastic participation of the men in this initial study cohort. We’re extremely grateful to them for being a vital part in bringing this potentially revolutionary treatment to market.”
SUI, or involuntary urinary leakage, affects an estimated 40 million Americans and 90 million Europeans, and occurs when the pressure in the bladder exceeds that of the muscle (the sphincter) around the urethra, caused by activities involving high intra-abdominal pressure, like coughing, laughing and exercising. SUI significantly impacts quality of life, as it can be debilitating, and often leads to depression, low self-esteem and social stigma.
About UroActive
UroActive is an active implantable electronic artificial urinary sphincter that is being developed to compensate for sphincter insufficiency in patients, both men and women, with SUI. It is based on a unique bionic platform using embedded smart, digital and robotic systems which, based on data collected from a patient, create a treatment algorithm that is specific for each patient’s needs. The UroMems technology platform is protected by more than 120 patents and is designed to overcome the limitations of current solutions by optimizing safety and performance, patient experience and surgeon convenience. STeP participation does not imply product authorization. UroActive has not received marketing authorization from the FDA and is not available for sale in the United States or in the EU.
For more information, please visit www.uromems.com.
Media Contact:
Shelli Lissick
651-276-6922
SOURCE UroMems

Nature Medicine Publication Highlights Potential for ONWARD® ARC Therapy™ to Improve Mobility After Parkinson’s Disease
Nature Medicine Publication Highlights Potential for ONWARD® ARC Therapy™ to Improve Mobility After Parkinson’s Disease
Therapy to be supported by newly awarded $1M grant from Michael J. Fox Foundation
EINDHOVEN, the Netherlands — November 06, 2023 — ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative spinal cord stimulation therapies to restore movement, function, and independence in people with spinal cord injury (SCI), today announces a publication in Nature Medicine using investigational ONWARD ARC Therapy to address gait challenges related to Parkinson’s disease.
The study participant described in the Nature Medicine publication has been living with Parkinson’s disease for nearly three decades. He has a severe gait disorder that has not responded to conventional therapies.
“I could hardly walk without frequent falls, several times a day. In certain situations, like getting into an elevator, I would stomp, freeze as they say,” said the study participant in a press release issued by Switzerland’s Lausanne University Hospital (CHUV).
In 2021, researchers from the Swiss Federal Institute of Technology (EPFL) and CHUV investigated the possibility that ONWARD ARC Therapy (precise, targeted electrical stimulation of the spinal cord) could address common side effects of Parkinson’s disease that negatively impact mobility. The team collaborated with Dr. Erwan Bezard, a renowned neuroscientist from France’s National Institute of Health and Medical Research (INSERM).
After the introduction of ARC Therapy and benefitting from a few weeks of rehabilitation, the study participant was able to walk without previously noticeable gait interruptions. Today, he uses ARC Therapy eight hours per day.
“I turn on the stimulation in the morning and turn it off in the evening. It allows me to walk better, to stabilize myself. Even stairs don’t scare me anymore. Every Sunday I go to the lake, and I walk about six kilometers. It’s awesome,” said the participant in the CHUV release.
“The breakthrough reported in Nature Medicine shows the remarkable potential to use the same technology platform and therapy we are developing for spinal cord injury to also address mobilitychallenges stemming from Parkinson’s disease,” says Dave Marver, CEO of ONWARD.
“It is impressive to see that by electrically stimulating the spinal cord the same way we have done in paraplegic patients, we may be able to address gait disorders due to Parkinson’s disease,” adds neurosurgeon Jocelyne Bloch, co-director with Professor G. Courtine of ONWARD research partner .NeuroRestore.
.NeuroRestore was awarded a $1 million grant from The Michael J. Fox Foundation for Parkinson’s Research (MJFF) to implant the ARC-IM System and investigate the effect of ARC Therapy in six additional participants with Parkinson’s disease. This study will assist ONWARD in determining whether to conduct additional clinical trials and potentially commercialize ARC Therapy in the future for those living with Parkinson’s disease.
“MJFF is committed to fulfilling the unmet needs of people living with Parkinson’s disease by ensuring the development of improved therapies,” said Katharina Klapper, Director of Clinical Research, MJFF. “The Foundation is pleased to award a grant for scientists at EPFL and ONWARD Medical to investigate the effects of ARC Therapy in people with experiencing gait challenges.”
All ONWARD devices and therapies, including but not limited to ARC-IM, ARC-EX, and ARC Therapy, are investigational and not available for commercial use.
Note: ONWARD has previously disclosed achievement of human proof-of-concept for Parkinson’s disease in its Company Presentation.
About ONWARD Medical
ONWARD is a medical technology company creating therapies to restore movement, function, and independence in people with spinal cord injury (SCI) and movement disabilities. Building on more than a decade of science and preclinical research conducted at leading neuroscience laboratories, the Company has received nine Breakthrough Device Designations from the US Food and Drug Administration for its ARC Therapy™ platform.
ONWARD® ARC Therapy, which can be delivered by external ARC-EX™ or implantable ARCIM™ systems, is designed to deliver targeted, programmed spinal cord stimulation. Positive results were presented in 2023 from the Company’s pivotal study, called Up-LIFT, evaluating the ability for transcutaneous ARC Therapy to improve upper extremity strength and function. The Company is now preparing regulatory approval submissions for ARC-EX for the US and Europe. In parallel, the Company is conducting studies with its implantable ARC-IM platform, which demonstrated positive interim clinical outcomes for improved blood pressure regulation, a component of hemodynamic stability following SCI. Other ongoing studies include combination use of ARC-IM with a brain-computer interface (BCI).
Headquartered in Eindhoven, the Netherlands, ONWARD has a Science and Engineering Center in Lausanne, Switzerland and a US office in Boston, Massachusetts. The Company also has an academic partnership with .NeuroRestore, a collaboration between the Swiss Federal Institute of Technology (EPFL), and Lausanne University Hospital (CHUV).
For more information, visit ONWD.com, and connect with us on LinkedIn and YouTube.
For Media Enquiries:
Aditi Roy, VP Communications
For Investor Enquiries:
Khaled Bahi, CFO
Disclaimer
Certain statements, beliefs, and opinions in this press release are forward-looking, which reflect the Company’s or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve several risks, uncertainties, and assumptions
that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties, and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, changes in demand, competition, and technology, can cause actual events, performance, or results to differ significantly from any anticipated development. Forward-looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions, or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release. All ONWARD devices and therapies referenced here, including but not limited to ARC-IM, ARC-EX, and ARC Therapy, are investigational and not available for commercial use.
Carisma Presents Pre-Clinical Proof of Concept for in vivo CAR-M using mRNA platform in collaboration with Moderna at SITC
Carisma Presents Pre-Clinical Proof of Concept for in vivo CAR-M using mRNA platform in collaboration with Moderna at SITC
- Data demonstrate feasibility, tolerability, and early efficacy of mRNA/LNP in vivo CAR-M therapy in pre-clinical models of metastatic solid tumors
- Proof of concept achieved for platform under the Moderna collaboration
- "In vivo CAR-M: Redirecting endogenous myeloid cells with mRNA for cancer immunotherapy" to be presented Friday, November 3, 2023, at 11:30 am PT
PHILADELPHIA, Oct. 31, 2023 /PRNewswire/ — Carisma Therapeutics Inc. (Nasdaq: CARM) (“Carisma” or the “Company”), a clinical stage biopharmaceutical company focused on discovering and developing innovative immunotherapies, today announced that it will present new findings at the Society for Immunotherapy of Cancer (SITC) 38th Annual Meeting regarding its first-of-its-kind collaboration with Moderna. The collaboration aims to bring together Carisma’s chimeric antigen receptor macrophage (CAR-M) platform with Moderna’s mRNA and lipid nanoparticle (LNP) technologies to generate and develop in vivo CAR-M therapeutics.
Accepted as a late-breaking presentation, “In vivo CAR-M: Redirecting endogenous myeloid cells with mRNA for cancer immunotherapy,” showcases data that demonstrate Carisma’s CAR-M therapy can be directly produced in vivo, or within the body, successfully redirecting endogenous myeloid cells against tumor-associated antigens using mRNA/LNP. The pre-clinical data demonstrate feasibility, tolerability, and efficacy against metastatic solid tumors. This novel approach to cancer immunotherapy offers an off-the-shelf solution that has the potential to increase access to CAR-based therapies and will be the basis of CAR-M programs to be developed under the Carisma and Moderna collaboration.
“The data presented at SITC is incredibly exciting as it demonstrates that we have the ability to make CAR-M directly in vivo with mRNA/LNP technology, leading to robust and targeted anti-tumor activity,” said Michael Klichinsky, PharmD, PhD, Co-Founder and Chief Scientific Officer at Carisma. “This off-the-shelf approach to treat cancer with engineered myeloid cells, developed in collaboration with Moderna, has the potential to transform the CAR field and be applied to numerous cancer targets and indications.”
“We are pleased to share data about the successful application of our mRNA platform to advance in vivo cell therapy,” said Lin Guey, PhD, Chief Scientific Officer of External Research Ventures at Moderna. “We look forward to the continuation of our pre-clinical work with Carisma and are optimistic that the joint scientific knowledge of both companies will accelerate the development of novel in vivo CAR-M therapies for patients.”
Details of Carisma data accepted for presentation at the SITC 38th Annual Meeting are as follows:
- “In vivo CAR-M: Redirecting endogenous myeloid cells with mRNA for cancer immunotherapy” presented on Friday, November 3, 2023, at 11:30 am PT
- “CAR-Macrophages with custom intronic shRNA exhibit enhanced efficacy against solid tumors” presented on Friday, November 3, 2023, 9:00 am – 7:00 pm PT
- “Engineered Microenvironment Converters (EM-C): Macrophages expressing synthetic cytokine receptors reverse immunosuppressive signals in solid tumors” presented on Friday, November 3, 2023, 9:00 am – 7:00 pm PT
- “A Phase 1, First in Human (FIH) study of autologous macrophages engineered to express an anti-HER2 chimeric antigen receptor (CAR) in participants (pts) with HER2 overexpressing solid tumors” presented on Friday, November 3, 2023, 9:00 am – 7:00 pm PT
Presentation and posters will be available on the SITC 38th Annual Meeting portal for registered attendees.
About Carisma
Carisma Therapeutics Inc. is a clinical stage biopharmaceutical company focused on utilizing our proprietary macrophage and monocyte cell engineering platform to develop transformative immunotherapies to treat cancer and other serious diseases. We have created a comprehensive, differentiated proprietary cell therapy platform focused on engineered macrophages and monocytes, cells that play a crucial role in both the innate and adaptive immune response. Carisma is headquartered in Philadelphia, PA. For more information, please visit www.carismatx.com.
About Moderna
In over 10 years since its inception, Moderna has transformed from a research-stage company advancing programs in the field of messenger RNA (mRNA), to an enterprise with a diverse clinical portfolio of vaccines and therapeutics across seven modalities, a broad intellectual property portfolio and integrated manufacturing facilities that allow for rapid clinical and commercial production at scale. Moderna maintains alliances with a broad range of domestic and overseas government and commercial collaborators, which has allowed for the pursuit of both groundbreaking science and rapid scaling of manufacturing. Most recently, Moderna’s capabilities have come together to allow the authorized use and approval of one of the earliest and most effective vaccines against the COVID-19 pandemic.
Moderna’s mRNA platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, and has allowed the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, cardiovascular diseases and auto-immune diseases. Moderna has been named a top biopharmaceutical employer by Science for the past eight years. To learn more, visit www.modernatx.com.
Cautionary Note on Forward-Looking Statements
Statements in this press release about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, statements relating to Carisma’s business, strategy, future operations, cash runway, the advancement of Carisma’s product candidates and product pipeline, and pre-clinical and clinical development of Carisma’s product candidates, including expectations regarding timing of initiation and results of pre-clinical and clinical trials. The words “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “goals,” “intend,” “may,” “might,” “outlook,” “plan,” “project,” “potential,” “predict,” “target,” “possible,” “will,” “would,” “could,” “should,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. For a discussion of these risks and uncertainties, and other important factors, any of which could cause Carisma’s actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” set forth in the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 10, 2023, as well as discussions of potential risks, uncertainties, and other important factors in Carisma’s other recent filings with the Securities and Exchange Commission. Any forward-looking statements that are made in this press release speak as of the date of this press release. Carisma undertakes no obligation to revise the forward-looking statements or to update them to reflect events or circumstances occurring after the date of this press release, whether as a result of new information, future developments or otherwise, except as required by the federal securities laws.
Media Inquiries:
Carisma to Present First Results From in vivo CAR-M Collaboration with Moderna at SITC 2023
Carisma to Present First Results From in vivo CAR-M Collaboration with Moderna at SITC 2023
-Late-breaking abstract to be presented on November 3rd at 11:30 am PT highlighting pre-clinical data with Moderna
-Two additional pre-clinical data abstracts highlighting next-generation enhancements to Carisma's cell therapy platform to be shared
PHILADELPHIA, Oct. 25, 2023 /PRNewswire/ — Carisma Therapeutics Inc. (Nasdaq: CARM) (“Carisma” or the “Company”), a clinical-stage biopharmaceutical company focused on discovering and developing innovative immunotherapies, today announced that new pre-clinical data leveraging an mRNA platform to develop in-vivo chimeric antigen receptor macrophage (“CAR-M”) will be presented as a late-breaking abstract (#LBA1514) at the upcoming Society for Immunotherapy of Cancer (SITC) 38th Annual Meeting held from Wednesday, November 1, to Sunday, November 5, 2023, in San Diego, California. Two posters highlighting next-generation enhancements to Carisma’s CAR-M platform, including a custom intronic shRNA approach and data on Engineered Microenvironment Converters (EM-C), will also be presented. Additionally, Carisma will share a trial-in-progress poster overviewing its Phase 1 first-in-human (FIH) study design of its lead program, CT-0508, sharing objectives and eligibility criteria.
Carisma will participate in the virtual SITC 2023 Annual Meeting Press Conference on Wednesday, November 1, 2023, from 12:00–1:30 pm PT.
“We are excited to present this pre-clinical data from our collaboration with Moderna for the first time,” said Steven Kelly, President and Chief Executive Officer of Carisma. “Over the past year, we have leveraged the expertise of both companies to pioneer the development of in-vivo mRNA/LNP-based cell therapy. The study is exploring the potential for an off-the-shelf treatment approach to enhance therapeutic benefit for patients living with cancer.”
Oral & Poster Presentations:
- In vivo CAR-M: Redirecting endogenous myeloid cells with mRNA for cancer immunotherapy
- Primary Author: Bindu Varghese, PhD
- Presentation Type/#: Oral – 1514
- Session Date/Time (PT): Friday, November 3, 2023, 11:30 am, Session 104: Late Breaking Abstract Session
- CAR-Macrophages with custom intronic shRNA exhibit enhanced efficacy against solid tumors
- Primary Author: Chris Sloas, PhD
- Presentation Type/#: Poster – 307
- Session Date/Time: Friday, November 3, 2023, 9:00 am – 7:00 pm
- Engineered Microenvironment Converters (EM-C): Macrophages expressing synthetic cytokine receptors reverse immunosuppressive signals in solid tumors
- Primary Author: Chris Sloas, PhD
- Presentation Type/#: Poster – 389
- Session Date/Time: Friday, November 3, 2023, 9:00 am – 7:00 pm
- A Phase 1, First in Human (FIH) study of autologous macrophages engineered to express an anti-HER2 chimeric antigen receptor (CAR) in participants (pts) with HER2 overexpressing solid tumors
- Primary Author: Kim Reiss, MD
- Presentation Type/#: Poster – 635
- Session Date/Time: Friday, November 3, 2023, 9:00 am – 7:00 pm
Presentation and posters will be available in the Journal for ImmunoTherapy of Cancer (JITC) supplement once published on Tuesday, October 31, 2023, at 9:00 am ET.
About CT-0508
CT-0508 is a human epidermal growth factor receptor 2 (HER2) targeted chimeric antigen receptor macrophage (CAR-M). It is being evaluated in a landmark Phase 1 multi-center clinical trial that focuses on patients with recurrent or metastatic HER2-overexpressing solid tumors whose cancers do not have approved HER2-targeted therapies or who do not respond to treatment. Carisma is selecting participants who have tumors of any anatomical origin, but with the commonality of overexpressing the HER2 receptor on the cell surface, which is the target for our CAR-M. The Phase 1 clinical trial marks the first time that engineered macrophages are being studied in humans. The trial continues to enroll patients at seven clinical sites in the U.S., including (i) Penn Medicine’s Abramson Cancer Center, (ii) the University of North Carolina Lineberger Comprehensive Cancer Center, (iii) the City of Hope National Medical Center, (iv) the MD Anderson Cancer Center, (v) the Sarah Cannon Cancer Research Institute, (vi) Oregon Health & Science University and (vii) Fred Hutchinson Cancer Center.
About Carisma
Carisma Therapeutics Inc. is a clinical-stage biopharmaceutical company focused on utilizing our proprietary macrophage and monocyte cell engineering platform to develop transformative immunotherapies to treat cancer and other serious diseases. We have created a comprehensive, differentiated proprietary cell therapy platform focused on engineered macrophages and monocytes, cells that play a crucial role in both the innate and adaptive immune response. Carisma is headquartered in Philadelphia, PA. For more information, please visit www.carismatx.com.
About Moderna
In over 10 years since its inception, Moderna has transformed from a research-stage company advancing programs in the field of messenger RNA (mRNA), to an enterprise with a diverse clinical portfolio of vaccines and therapeutics across seven modalities, a broad intellectual property portfolio and integrated manufacturing facilities that allow for rapid clinical and commercial production at scale. Moderna maintains alliances with a broad range of domestic and overseas government and commercial collaborators, which has allowed for the pursuit of both groundbreaking science and rapid scaling of manufacturing. Most recently, Moderna’s capabilities have come together to allow the authorized use and approval of one of the earliest and most effective vaccines against the COVID-19 pandemic.
Moderna’s mRNA platform builds on continuous advances in basic and applied mRNA science, delivery technology and manufacturing, and has allowed the development of therapeutics and vaccines for infectious diseases, immuno-oncology, rare diseases, cardiovascular diseases and auto-immune diseases. Moderna has been named a top biopharmaceutical employer by Science for the past eight years. To learn more, visit www.modernatx.com.
Cautionary Note on Forward-Looking Statements
Statements in this press release about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, statements relating to Carisma’s business, strategy, future operations, cash runway, the advancement of Carisma’s product candidates and product pipeline, and clinical development of Carisma’s product candidates, including expectations regarding timing of initiation and results of pre-clinical and clinical trials. The words “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “goals,” “intend,” “may,” “might,” “outlook,” “plan,” “project,” “potential,” “predict,” “target,” “possible,” “will,” “would,” “could,” “should,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. For a discussion of these risks and uncertainties, and other important factors, any of which could cause Carisma’s actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” set forth in the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 10, 2023, as well as discussions of potential risks, uncertainties, and other important factors in Carisma’s other recent filings with the Securities and Exchange Commission. Any forward-looking statements that are made in this press release speak as of the date of this press release. Carisma undertakes no obligation to revise the forward-looking statements or to update them to reflect events or circumstances occurring after the date of this press release, whether as a result of new information, future developments or otherwise, except as required by the federal securities laws.
Media Inquiries:
Immatics Receives FDA Regenerative Medicine Advanced Therapy (RMAT) Designation for ACTengine® IMA203 TCR-T Monotherapy
Immatics Receives FDA Regenerative Medicine Advanced Therapy (RMAT) Designation for ACTengine® IMA203 TCR-T Monotherapy
- RMAT designation granted by FDA CBER for IMA203 cell therapy in multiple PRAME-expressing tumors including cutaneous and uveal melanoma, ovarian cancer and other cancer types
- Regulatory activities underway with an initial focus on a registration-directed trial in melanoma as step one to leverage the full breadth of PRAME
Houston, Texas and Tuebingen, Germany, October 24, 2023 – Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, today announced that its IMA203 TCR-T program has received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA Center for Biologics Evaluation and Research (CBER) in multiple relapsed and/or refractory HLA-A*02:01-positive and PRAME-expressing cancers, including cutaneous melanoma, uveal melanoma, endometrial carcinoma, synovial sarcoma, and ovarian cancer. IMA203 is a TCR-T cell therapy targeting PRAME, a protein frequently expressed in a large variety of solid tumors.
“The FDA RMAT designation for multiple indications underscores the broad potential of IMA203 and the benefits it may provide for advanced-stage solid tumor patients. This is an important regulatory milestone and a recognition of our clinical development progress for this program,” said Cedrik Britten, Chief Medical Officer of Immatics. “The close support from the FDA resulting from the RMAT status enhances our efforts to accelerate bringing IMA203 to cancer patients by enabling real-time discussions on patient populations, trial design and CMC.”
Established under the 21st Century Cures Act, RMAT designation is a dedicated program designed to expedite the development and review processes for promising pipeline products, including cell therapies, that includes all the benefits of Fast Track and Breakthrough designation programs. An investigational cell therapy is eligible for RMAT designation if it meets the definition of regenerative medicine therapy, it is intended to treat, modify, reverse, or cure a serious or life-threatening disease; and preliminary clinical evidence indicates that the therapy has the potential to address unmet medical needs for that disease. Advantages of the RMAT designation include early interactions with the FDA that may be used to discuss potential surrogate or intermediate endpoints for accelerated approval and potential ways to satisfy post-approval requirements, potential priority review of the biologics license application (BLA) and other opportunities to expedite development and review.
Based on publicly available information1, it is the Company’s understanding that this is the first time that FDA has granted a RMAT designation for an oncology drug candidate for more than two solid tumor indications. As of Sep 30, 2023, the U.S. FDA has received at least 238 requests for RMAT designations and granted 922.
About IMA203 and target PRAME
ACTengine® IMA203 T cells are directed against an HLA-A*02:01-presented peptide derived from preferentially expressed antigen in melanoma (PRAME), a protein frequently expressed in a large variety of solid cancers, thereby supporting the program’s potential to address a broad cancer patient population. Immatics’ PRAME peptide is present at a high copy number per tumor cell and is homogeneously and specifically expressed in tumor tissue. The peptide has been identified and characterized by Immatics’ proprietary mass spectrometry-based target discovery platform, XPRESIDENT®. Through its proprietary TCR discovery and engineering platform XCEPTOR®, Immatics has generated a highly specific T cell receptor (TCR) against this target for its TCR-based cell therapy approach, ACTengine® IMA203.
ACTengine® IMA203 TCR-T is currently being evaluated in three ongoing Phase 1b dose expansion cohorts in last-line patients: Cohort A IMA203 GEN1 monotherapy, Cohort B IMA203 in combination with an immune checkpoint inhibitor (deprioritized) and Cohort C IMA203CD8 GEN2 monotherapy, where IMA203 engineered T cells are co-transduced with a CD8αβ co-receptor.
About ACTengine®
ACTengine® is a personalized cell therapy approach for patients with advanced solid tumors. The patient’s own T cells are genetically engineered to express a novel, proprietary TCR directed against a defined cancer target. The modified T cells are then reinfused into the patient to attack the tumor. The approach is also known as TCR-engineered cell therapy (TCR-T). All Immatics’ ACTengine® product candidates can be rapidly manufactured utilizing a proprietary manufacturing process designed to enhance T cell engraftment and persistence in vivo.
The ACTengine® T cell products are manufactured at the Evelyn H. Griffin Stem Cell Therapeutics Research Laboratory in collaboration with UTHealth.
– END –
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
Immatics intends to use its website www.immatics.com as a means of disclosing material non-public information. For regular updates, you can also follow us on Twitter, Instagram and LinkedIn.
Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the timing of product candidates and Immatics’ focus on partnerships to advance its strategy are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
| Media | |
| Eva Mulder or Charlotte Spitz | |
| Trophic Communications | |
| Phone: +31 65 2331 579 | |
| Investor Relations | |
| Sabrina Schecher, Ph.D. | |
| Senior Director, Investor Relations | |
| Phone: +49 89 262002433 | |
1 Source: https://bioinformant.com/rmat/
2 Source: FDA – https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/cumulative-cber-regenerative-medicine-advanced-therapy-rmat-designation-requests-received-fiscal
MinervaX Raises EUR 54M in Upsized Financing to Advance the Development of its Maternal Vaccine Against Group B Streptococcus
MinervaX Raises EUR 54M in Upsized Financing to Advance the Development of its Maternal Vaccine Against Group B Streptococcus
- New investment from EQT Life Sciences and OrbiMed with participation from existing investors
- MinervaX will hold financial reserves of more than €125 million, following the financing
- The financing supports the Company’s efforts to commence a Phase III clinical trial of its Maternal Vaccine against Group B Streptococcus
Copenhagen, Denmark, 11 October 2023 – MinervaX ApS, a privately held Danish biotechnology company developing a novel, prophylactic vaccine against Group B Streptococcus (GBS), has today announced the completion of a EUR 54 million upsized financing. The financing includes investment from new investors EQT Life Sciences and OrbiMed, with participation from existing investors Novo Holdings, Pureos Ventures, Sanofi Ventures, Trill Impact Ventures, Adjuvant Capital, Wellington Partners, Industrifonden, Sunstone LifeScience Ventures, and LF Invest. Vincent Brichard of EQT Life Sciences and Tal Zaks of OrbiMed will join the MinervaX Board of Directors.
GBS is a leading cause of life-threatening infections in newborns as well as adverse pregnancy outcomes such as preterm delivery and stillbirths. Current prophylactic measures provide insufficient protection, meaning there is an urgent need to accelerate the development of a GBS vaccine. A video describing the unmet medical need for a GBS vaccine and details of MinervaX’s novel GBS maternal vaccine can be found on the Company’s website, here: https://youtu.be/HvkRJBqsjjY.
The Company is currently progressing two Phase II clinical trials in 470 pregnant persons across Denmark, United Kingdom, Uganda and South Africa. Initial data from these clinical trials are highly positive and demonstrate that the vaccine has an acceptable safety profile, is highly immunogenic and gives rise to functionally active antibodies. Details of MinervaX’s clinical trials can be found at clinicaltrials.gov under the identifiers NCT04596878 and NCT05154578. In addition to pregnant persons, MinervaX is also pursuing Phase I development of its novel GBS vaccine in Older Adults, under identifier NCT05782179.
This financing will enable MinervaX to progress its novel GBS vaccine towards Phase III clinical trials in 2024.
Per Fischer, CEO of MinervaX, said: “The addition of EQT Life Sciences and OrbiMed to our existing investor consortium further strengthens the Company’s resolve to advance our novel GBS vaccine towards Phase III clinical trials in pregnant persons. It also provides additional validation and recognition of the acceptable safety profile and strong data demonstrated in the Phase II clinical trials. We are delighted to welcome Vincent Brichard and Tal Zaks to the board of directors, who will bring invaluable vaccine expertise as we continue to address the pressing need for the development of a novel vaccine to address the unmet medical burden of Group B Streptococcus.”
Vincent Brichard, Venture Partner EQT Life Sciences, commented: “EQT Life Sciences is thrilled to take an active part in the MinervaX prophylactic vaccine against GBS with the hope to save newborns’ lives. We are impressed by the clinical data achieved so far, the quality of the team and the near-term milestones enabling MinervaX to start a registration trial.”
Tal Zaks, Partner at OrbiMed, added: “We recognize the unmet need for better protection against GBS disease for vulnerable populations and the potential for MinervaX’s vaccine to provide best-in-class efficacy. I look forward to working with the MinervaX team to support the full development of this program.”
ENDS
For further information please contact:
MinervaX
Per Fischer | Chief Executive Officer
Email:
Optimum Strategic Communications
Mary Clark / Stephen Adams/ Zoe Bolt
Email:
Tel: +44 (0) 203 882 9621
Notes to Editors:
About MinervaX
MinervaX is a Danish biotechnology company, established in 2010 to develop a prophylactic vaccine against Group B Streptococcus (GBS), based on research from Lund University. MinervaX is developing a GBS vaccine for maternal immunization, and now also for vaccination of older adults, with Phase II data suggesting superior efficacy compared with other GBS vaccine candidates in development. The latter are based on traditional capsular polysaccharide (CPS) conjugate technology. By contrast, MinervaX’s vaccine is a protein-only vaccine based on fusions of highly immunogenic and protective protein domains from selected surface proteins of GBS (the Alpha-like protein family). Given the broad distribution of proteins contained in the vaccine on GBS strains globally, it is expected that MinervaX’s vaccine will confer protection against virtually 100% of all GBS isolates. www.minervax.com
About Group B Streptococcus (GBS)
GBS is responsible for nearly 50% of all life-threatening infections in newborns. At any given time, some 15-25% of women are spontaneously colonized with GBS, and they run the risk of transmitting the bacteria to their child in the womb, during birth and/or during the first months of life. GBS colonization may lead to late abortions, premature delivery, or stillbirth and, in the newborn child, may result in sepsis, pneumonia or meningitis, all of which carry a significant risk of severe morbidity, long- term disability or death.
Currently, the only preventative strategy available involves the use of intravenously delivered prophylactic antibiotics which cannot comprehensively prevent GBS infection in utero and do not protect against late-onset infection in newborns. Not only is this approach expensive and logistically challenging, it fails to cover all, including the most severe cases in the US and Europe, and is rarely available in resource- limited settings. Finally, it carries the risk for increasing antibiotics resistance, a recognized worldwide health threat.
The development of a GBS vaccine is also endorsed by Group B Strep Support and Group B Strep International, and GBS has been prioritized by several public health organizations including the WHO. Both increased uptake of immunization among pregnant women and greater awareness of the implications of GBS suggest that a safe and effective vaccine targeting GBS would be well suited to address this unmet need.
About EQT Life Sciences
EQT Life Sciences was formed in 2022 following an integration of LSP, a leading European life sciences and healthcare venture capital firm, into the EQT platform. As LSP, the firm raised over EUR 3.0 billion (USD 3.5 billion) and supported the growth of more than 150 companies since it started to invest over 30 years ago. With a dedicated team of highly experienced investment professionals, coming from backgrounds in medicine, science, business, and finance, EQT Life Sciences backs the smartest inventors who have ideas that could truly make a difference for patients.
More info: www.eqtgroup.com
Follow EQT on LinkedIn, Twitter, YouTube and Instagram
About OrbiMed
OrbiMed is a healthcare investment firm, with approximately $17 billion in assets under management. OrbiMed invests globally across the healthcare industry through a range of private equity funds, public equity funds, and royalty/credit funds. OrbiMed’s team of over 100 professionals is based in New York City, San Francisco, Shanghai, Hong Kong, Mumbai, Herzliya, London and other key global markets.
More info: www.orbimed.com