NEUWAY Pharma and WACKER Launch Research Project to Develop RNA-Based Actives for the Treatment of Central Nervous System Diseases
Joint Press Release by WACKER and NEUWAY Pharma
Munich and Bonn, Oct 12, 2022- Bonn-based biotech company NEUWAY Pharma and Munich-based chemical company WACKER have launched a research project to identify and manufacture RNA-based actives for the treatment of diseases of the central nervous system (CNS). They will make use of NEUWAY’s protein-based drug delivery technology EnPC®. EnPC® allows for targeted drug delivery via the blood-brain barrier into the CNS.
Under the collaboration agreement, NEUWAY Pharma and WACKER intend to jointly research active substances based on ribonucleic acid (RNA) for the treatment of CNS-related diseases; the companies will also study related manufacturing processes. They will make use of NEUWAY Pharma’s protein-based drug delivery technology EnPC® (Engineered Protein Capsules), where active ingredient molecules are encapsulated by protein capsules and thereby transported across the blood-brain barrier – which is difficult to pass – into the CNS.
The blood-brain barrier serves as natural protection against harmful substances. Crossing it has so far posed a major challenge for the treatment of CNS diseases. EnPC® now makes effective transport of drugs into the CNS possible. This technology also offers a range of advantages for RNA-related therapies. Alongside lower costs in manufacturing, dosing of drugs based on EnPC® can be handled flexibly, for instance. Intravenous administration is furthermore possible, which eases the burden on clinicians, healthcare providers, payors and patients.
Within the research project, WACKER will take on the production and analysis of various mRNA grades, in particular mRNA – mRNA (messenger RNA) is a special type of RNA made from DNA. NEUWAY is responsible for manufacturing the EnPCs, the encapsulation and the design of selected RNA compounds, as well as associated (bio-)analysis and investigation of therapeutic relevance, both in vitro and in vivo.
“We see great opportunities in the joint research project with WACKER. WACKER’s expertise in the production of mRNA supports our strategy of advancing transformative neuropharmaceuticals for the treatment of diseases of the central nervous system,” says Oliver Ernst, CEO and managing director of NEUWAY Pharma. “We are pleased that, with our expertise, we can contribute to research into new treatment methods for diseases of the central nervous system. The combination of mRNA-based drugs with NEUWAY’s delivery system has great therapeutic potential,” says Hagen Richter, who is responsible for research in the area of nucleic acids at WACKER. The molecular biologist is in charge of the project on WACKER’s side.
About NEUWAY Pharma
NEUWAY Pharma is a German biotech company based in Bonn. It is developing an entirely novel class of biotherapeutics, based on its patent-protected Engineered Protein Capsules (EnPC®). These can cross the blood-brain barrier and deliver innovative neuropharmaceuticals for the treatment of diseases of the central nervous system (CNS). Find out more at www.neuway-pharma.com.
About WACKER
Wacker Chemie AG is a global company with state-of-the-art specialty chemical products found in countless everyday items, ranging from cosmetic powders to solar cells. WACKER’s portfolio comprises more than 3,200 products supplied in over 100 countries. WACKER has a global network of 27 production sites, 23 technical competence centers and 52 sales offices. In 2021, the Group’s 14,400 employees generated global sales of €6.21 billion. Wacker Chemie AG is listed on the Deutsche Boerse Prime Standard and on the MDAX (ISIN: DE000WCH8881). Find out more at www.wacker.com.
Contact
Wacker Chemie AG
Manuela Dollinger
Tel.: +49 89 6279-1629
NEUWAY Pharma
Christine Kuhn
Tel.: +49 228-522198-0
Immatics Reports Interim Clinical Data Update on ACTengine® IMA203 TCR-T Monotherapy Targeting PRAME
Immatics Reports Interim Clinical Data Update on ACTengine® IMA203 TCR-T Monotherapy Targeting PRAME
Immatics Reports Interim Clinical Data Update on ACTengine® IMA203 TCR-T Monotherapy Targeting PRAME
- Clinical validation of PRAME as multi-tumor target with large potential for TCR-based therapies: confirmed responses in different solid cancers, in patients with high and low PRAME expression
- Update covers data from 27 patients in completed Phase 1a dose escalation and first 5 patients in Phase 1b dose expansion (cohort A) treated with IMA203 monotherapy
- Confirmed objective response rate (cORR): 50% (6/12) at target dose or above with at least 1 billion infused TCR-T cells across Phase 1a and 1b; thereof 80% cORR (4/5) in Phase 1b patients alone with all responses ongoing at data cut-off
- Confirmed responses across different solid tumor types: cutaneous melanoma, ovarian cancer, head and neck cancer, uveal melanoma, and synovial sarcoma
- Treatment with IMA203 continues to show manageable tolerability; biological data including T cell engraftment, persistence and tumor infiltration consistent with clinical data
- IMA203 TCR-T is part of Immatics’ strategy to leverage the full clinical potential of targeting PRAME; next data read-outs on IMA203 monotherapy, IMA203 in combination with a checkpoint inhibitor and 2nd generation IMA203CD8 planned during 2023
Houston, Texas and Tuebingen, Germany, October 10, 2022 – Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, today announced a clinical data update for the IMA203 monotherapy covering the completed Phase 1a dose escalation part of the trial and initial data from the first 5 patients in the ongoing Phase 1b dose expansion cohort A (monotherapy). In the Phase 1 trial with ACTengine® IMA203, Immatics is treating recurrent and/or refractory solid cancer patients utilizing TCR-T cells directed against an HLA-A*02-presented peptide derived from PRAME, which is frequently expressed across several solid cancer indications. Overall, IMA203 continues to be well tolerated and achieved confirmed objective responses across multiple solid cancers such as cutaneous melanoma, ovarian cancer, head and neck cancer, uveal melanoma, and synovial sarcoma. Encouraging early signs of improved durability were seen with a 50% (6/12) confirmed objective response rate, when patients were infused at the target dose or above with more than 1 billion TCR-T cells.
Key clinical findings from IMA203 TCR-T monotherapy
The data obtained during the Phase 1a and Phase 1b cohort A trial provide clinical validation of PRAME as a highly promising T cell target for solid cancers. Confirmed clinical responses were observed at high and low PRAME-expression levels above threshold, indicating IMA203’s potential to provide clinical benefit for all PRAME biomarker-positive cancer patients. The predicted high PRAME prevalence across key indications has so far been supported by prevalence rates obtained during the clinical screening of patients.
Moving from Phase 1a to Phase 1b, Immatics has continued to introduce planned improvements that may influence clinical outcomes including (1) applying higher cell doses (DL4 and exploratory DL5), (2) optimizing the cell product through manufacturing enhancements and (3) working with disease area experts to gradually reduce the fraction of very heavily pre-treated patients with extreme tumor burden who have exhausted standard of care and have undergone multiple clinical trials. In addition, the focus in Phase 1b is also shifting from initial objective response rate (ORR) determined at the ~6-week scan to confirmed ORR determined at the ~12-week scan.
Preliminary Objective Response Rates (ORR; RECIST 1.1) in Phase 1a and Phase 1b Cohort A
| Phase 1a | Phase 1a + Phase 1b | Phase 1b only | ||
| All pts (DL1-4) | DL4 pts only 1 | DL4/DL5 pts only1 | All pts (DL4/DL5)1 | |
| Patients Treated | 27 | 7 | 12 | 5 |
| ORR (~week 6) | 48% (13/27) | 57% (4/7) | 67% (8/12) | 80% (4/5) |
| cORR (~week 12)2 | 19% (5/27) | 29% (2/7) | 50% (6/12)* | 80% (4/5)* |
1 All patients received >1 billion total TCR-T cells; 2confirmed ORR (cORR), * 1 patient with SD at ~6-week scan with pending ~12-week scan considered as non-responder for cORR; DL – dose level
Positively evolving durability profile for IMA203 was observed at higher doses: 6 of 12 patients (50%) treated with more than 1 billion infused TCR-T cells (DL4 and DL5) in the Phase 1a and Phase 1b cohort A part of the trial experienced a confirmed objective response (partial response according to RECIST 1.1). In the Phase 1b part of the trial alone, 4 of 5 patients (80%) had a confirmed objective response which were all ongoing at the timepoint of data cut-off.
“The data presented today highlight the clinical potential of PRAME as one of the most promising multi-tumor targets to achieve meaningful benefits for a large cancer patient population,” commented Cedrik Britten, MD, Chief Medical Officer at Immatics. “In addition to this first data from IMA203 monotherapy today, we are awaiting data from two additional dose expansion cohorts: IMA203 together with an immune checkpoint inhibitor and our 2nd generation product candidate IMA203CD8. As we continue to shift our focus from Phase 1a to Phase 1b, we look forward to reporting meaningful data throughout 2023, including safety and response rates, as well as durability of response with a longer follow-up time. In addition, we are excited to start a first-in-human trial with our half-life extended Bispecific against PRAME, TCER® IMA402, also in 2023.”
Safety data for IMA203 monotherapy across Phase 1a and Phase 1b: Treatment with IMA203 continues to show manageable tolerability profile.
- At data cut-off on September 6, 2022, 32 patients were infused with IMA203 TCR-T cells.
- Most frequent treatment-emergent adverse events (TEAEs) were as expected for cell therapies.
- All patients experienced expected cytopenia (Grade 1-4) associated with lymphodepletion. 31 patients (97%) experienced cytokine release syndrome (CRS) of any grade: 29 patients had low to moderate (Grade 1-2), and 2 patients had Grade 3 CRS that occurred in Phase 1a; both recovered to Grade ≤2 after 3 and 4 days. 5 patients (16%) experienced a low to moderate (Grade 1-2) immune effector cell associated neurotoxicity syndrome (ICANS). No dose-dependent increase of CRS and ICANS was observed.
- No additional dose limiting toxicities (DLT) were observed since the initial data release in March 2021.
Phase 1a – Clinical activity: IMA203 demonstrated a high initial objective response rate in several solid tumor types.
- At data cut-off on September 6, 2022, a total of 27 patients received IMA203 monotherapy in the Phase 1a dose escalation trial:
- High initial objective response rate (ORR; partial responses according to RECIST 1.1) of 48% (13/27) was observed at the first CT scan post infusion at ~week 6, and a confirmed ORR of 19% (5/27) the second CT scan at ~week 12.
- 7 out of 27 patients received doses above 1 billion TCR-T cells (DL4); initial ORR was 57% (4/7) and confirmed ORR was 29% (2/7) in these patients.
- Patients were heavily pre-treated with a mean of 4.2 lines of prior systemic treatment and a particularly high baseline tumor burden.
- The provisional recommended Phase 2 dose (RP2D) for Phase 1b dose expansion was determined to be DL4.
Phase 1b Cohort A – Clinical activity: IMA203 monotherapy demonstrates high confirmed objective response rate of 80% with early signs of prolonged durability.
- At data cut-off on September 6, 2022, 5 patients received IMA203 monotherapy at DL4 and DL5 in the Phase 1b cohort A dose expansion trial:
- 4 out of 5 patients (80%) experienced an initial objective response at ~week 6 (PR according to RECIST 1.1).
- In all 4 patients, objective responses were confirmed at ~week 12 and were ongoing at data cut-off: confirmed ORR was 80% (4/5).
- All 4 responses were observed in different solid tumor types: cutaneous melanoma, ovarian cancer, uveal melanoma and head and neck cancer.
- Patients were heavily pre-treated with a mean of 4.0 lines of prior systemic treatment and high to moderate baseline tumor burden.
ACTengine® IMA203 is currently being evaluated in an ongoing Phase 1b study including three expansion cohorts: (A) IMA203 as a monotherapy, (B) IMA203 in combination with an immune checkpoint inhibitor and (C) IMA203CD8, a next-generation cell therapy where IMA203 engineered T cells are co-transduced with a CD8αβ co-receptor. Further data read-outs on the individual cohorts are planned throughout 2023. In addition to the ACTengine® programs, Immatics is addressing PRAME-positive cancers with a second therapeutic modality: TCR Bispecifics. The company’s TCER® IMA402 is a next-generation, half-life extended TCR Bispecific which will enter the clinic in 2023. Both approaches, ACTengine® and TCER®, are distinct therapeutic modalities that have the potential to provide innovative treatment options for a variety of cancer patient populations with different medical needs.
Immatics conference call
Immatics will host a conference call today, October 10, 2022, at 8:30 am EDT / 2:30 pm CEST to discuss these clinical data. The webcast and presentation can be accessed directly through this link. Participants may also access the slides and the webcast on the Immatics website in the Investors section under “Presentations” at www.investors.immatics.com/events-presentations. A replay of the webcast will be made available shortly after the conclusion of the call and archived on the Company’s website for at least 90 days.
About IMA203 and target PRAME
ACTengine® IMA203 T cells are directed against an HLA-A*02-presented peptide derived from preferentially expressed antigen in melanoma (PRAME), a protein frequently expressed in a large variety of solid cancers, thereby supporting the programs’ potential to address a broad cancer patient population. Immatics’ PRAME peptide is present at a high copy number per tumor cell and is homogenously and specifically expressed in tumor tissue. The peptide has been identified and characterized by Immatics’ proprietary mass spectrometry-based target discovery platform XPRESIDENT®. Through its proprietary TCR discovery and engineering platform XCEPTOR®, Immatics has generated a highly specific T cell receptor (TCR) against this target for its TCR-based cell therapy approach, ACTengine® IMA203.
About ACTengine®
ACTengine® is a personalized cell therapy approach for patients with advanced solid tumors. The patient’s own T cells are genetically engineered to express a novel, proprietary TCR directed against a defined cancer target. The modified T cells are then reinfused into the patient to attack the tumor. The approach is also known as TCR-engineered cell therapy (TCR-T). All Immatics’ ACTengine® product candidates can be rapidly manufactured utilizing a proprietary manufacturing process designed to enhance T cell engraftment and persistence in vivo.
The ACTengine® T cell products are manufactured at the Evelyn H. Griffin Stem Cell Therapeutics Research Laboratory in collaboration with UTHealth. The ACTengine® Programs are co-funded by the Cancer Prevention and Research Institute of Texas (CPRIT).
– END –
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
For regular updates about Immatics, visit www.immatics.com. You can also follow us on Twitter and LinkedIn.
Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the timing of product candidates and Immatics’ focus on partnerships to advance its strategy are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
| Media and Investor Relations Contact | |
| Jacob Verghese or Eva Mulder | |
| Trophic Communications | |
| Phone: +49 89 2070 89831 or +31 65 2331 579 | |
| Immatics N.V. | |
| Anja Heuer | Jordan Silverstein |
| Director, Corporate Communications | Head of Strategy |
| Phone: +49 89 540415-606 | Phone: +1 281 810 7545 |
Immatics Announces $110 Million Underwritten Offering of Ordinary Shares
Immatics Announces $110 Million Underwritten Offering of Ordinary Shares
Immatics Announces $110 Million Underwritten Offering of Ordinary Shares
Houston, Texas and Tuebingen, Germany, October 10, 2022 – Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, announced today that it has agreed to sell, by way of an underwritten public offering, 10,905,000 of its ordinary shares at a price of $10.09 per share. The gross proceeds from the offering, before deducting the underwriting discount and offering expenses, are expected to be approximately $110 million. The offering is expected to close on October 12, 2022, subject to customary closing conditions.
The offering included participation from investors including Armistice Capital Master Fund Ltd., Dellora Investments, EcoR1 Capital, Nantahala Capital, Perceptive Advisors, Rock Springs Capital, RTW Investments, LP, Samsara BioCapital, SilverArc Capital, Sofinnova Investments, Wellington Management, 683 Capital and other specialist biotech investors.
Jefferies and SVB Securities are acting as joint book-running managers for the offering.
A registration statement relating to these securities has been filed with the U.S. Securities and Exchange Commission (the “SEC”) and was declared effective on August 9, 2021. The offering is being made only by means of a prospectus supplement and accompanying prospectus. When available, copies of the final prospectus supplement and accompanying prospectus related to the offering may be obtained for free from Jefferies LLC, Attention: Equity Syndicate Prospectus Department, 520 Madison Avenue, 2nd Floor, New York, NY 10022, telephone: (877) 821-7388, email: ; or SVB Securities LLC, Attention: Syndicate Department, 53 State Street, 40th Floor, Boston, MA 02109, telephone: (800) 808-7525, ext. 6105, email: .
This press release shall not constitute an offer to sell or a solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Any offers, solicitations or offers to buy, or any sales of securities will be made in accordance with the registration requirements of the Securities Act of 1933, as amended.
– END –
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the closing of the public offering and gross proceeds therefrom are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements.
For more information, please contact:
| Media and Investor Relations Contact | |
| Jacob Verghese or Eva Mulder | |
| Trophic Communications | |
| Phone: +49 89 2070 89831 or +31 65 2331 579 | |
| Immatics N.V. | |
| Anja Heuer | Jordan Silverstein |
| Director, Corporate Communications | Head of Strategy |
| Phone: +49 89 540415-606 | Phone: +1 281 810 7545 |
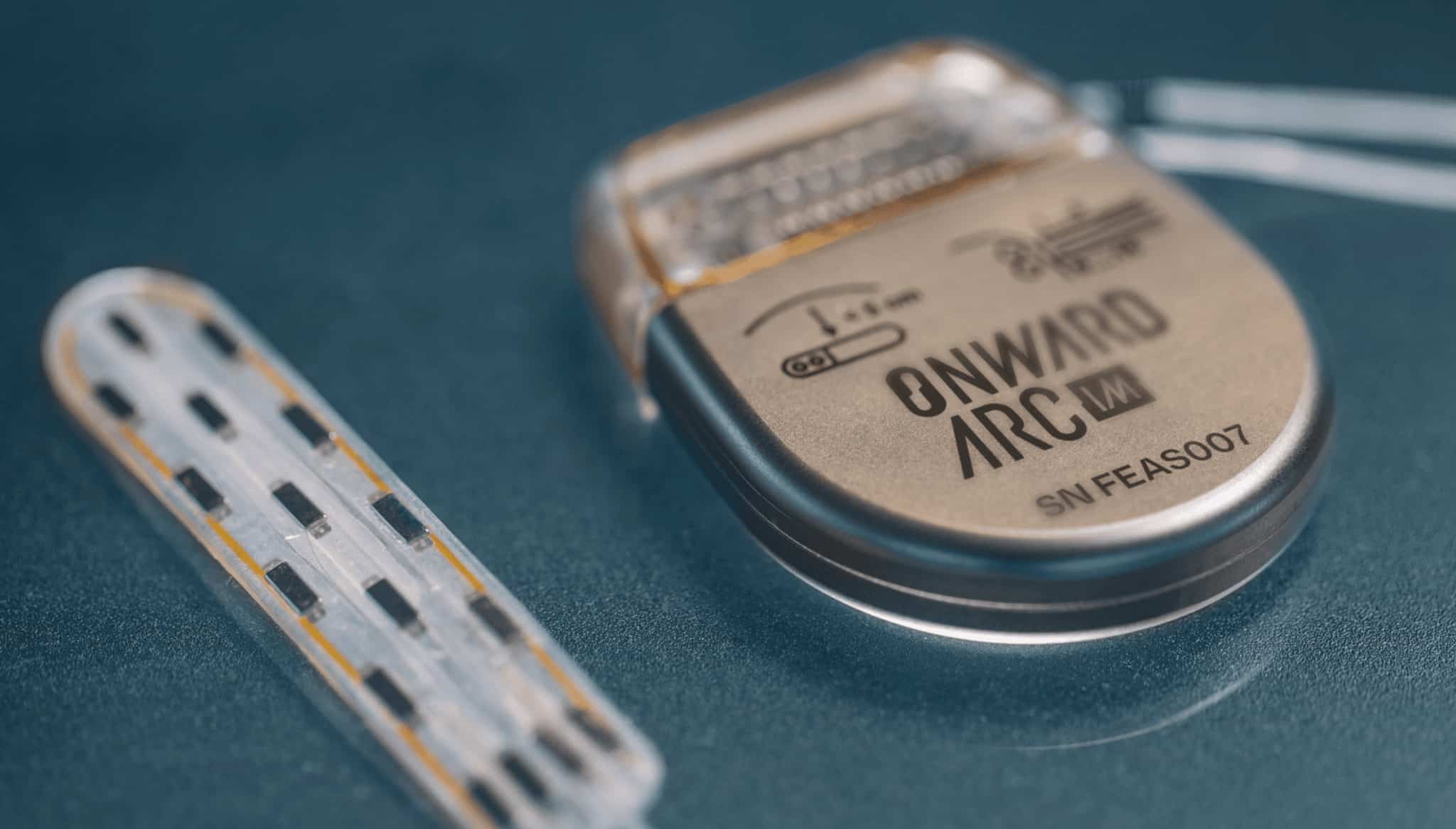
ONWARD Announces Appointment of Vivian Riefberg to Board of Directors
ONWARD Announces Appointment of Vivian Riefberg to Board of Directors
EINDHOVEN, the Netherlands, LAUSANNE, Switzerland, and BOSTON, MA USA—September 26, 2022—ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury, today announced the appointment of Vivian Riefberg as a non-executive member of its Board of Directors. Ms. Riefberg will join the Board immediately and will serve on its Compensation Committee. Her appointment to the Board will be presented for shareholder approval at the next Annual General Meeting.
“Vivian Riefberg is a distinguished expert in health care, government, and strategy. I am excited and honored to welcome her to our Board”, said Dave Marver, Chief Executive Officer. “Vivian brings a rich understanding of the institutions with which we will collaborate to bring our novel therapies to market, including the U.S. Veterans Health Administration. Her experience and insights will be invaluable as we work to create an entirely new therapeutic domain in neuromodulation and help people with spinal cord injury and other movement disabilities.”
“ONWARD has the opportunity to build a very special enterprise that is both successful and impactful”, said Vivian Riefberg. “I have had the privilege to counsel many leading companies during my career. ONWARD has a chance to help people with spinal cord injury by leveraging groundbreaking science, technology, and intellectual property, fueled by a fullhearted mission.”
In 2020, Ms. Riefberg retired as a senior partner with McKinsey & Company, where she held a variety of senior positions, including leader of the Public Sector Practice for the Americas and co- leader of the U.S. Health Care practice. She served on McKinsey & Company’s global board of directors and on the committee evaluating and developing global partners. Ms. Riefberg is currently Professor of Practice at the University of Virginia Darden School of Business where she holds the David C. Walentas Jefferson Scholars Chair. At Darden, Ms. Riefberg focuses on healthcare, consulting, and strategy across the private, public and not-for-profit sectors. Her emphasis is senior executive leadership, including leading during times of uncertainty and crisis, solutions and innovations in healthcare, and women’s leadership.
Ms. Riefberg serves on the Board of Signify Health, K Health and Lightrock, an impact investing firm, as well as of the Public Broadcasting System (PBS), Johns Hopkins Medicine, the Lorna Breen Heroes Foundation and the National Education Equity Lab. She is also an advisory board member for the Smithsonian’s planned American Women’s History Museum. She previously served on the U.S. National Institutes of Health (NIH) Clinical Center Board of Governors and on the NIH Advisory Board for Clinical Research. She also served on the Board of Directors of the Partnership for a Healthier America (PHA), a nonprofit, created to mobilize efforts to solve the child obesity challenge as an outgrowth of First Lady Michelle Obama’s Let’s Move campaign.
Ms. Riefberg is a frequent speaker at various conferences including the World Economic Forum at Davos. She is also a contributor to leading industry publications on improving U.S. healthcare, addressing government productivity and women’s leadership.
She holds a B.A., magna cum laude in history from Harvard-Radcliffe College and an M.B.A. with distinction from Harvard Business School.
About ONWARD Medical
ONWARD is a medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injuries. ONWARD’s work builds on more than a decade of basic science and preclinical research conducted at the world’s leading neuroscience laboratories. ONWARD’s ARC Therapy, which can be delivered by implantable (ARC-IM) or external (ARC-EX) systems, is designed to deliver targeted, programmed spinal-cord stimulation to restore movement and other functions in people with spinal cord injury, ultimately improving their quality of life.
ONWARD has received three Breakthrough Device Designations from the U.S. FDA encompassing both ARC-IM and ARC-EX. ARC-EX is an external, non-invasive platform consisting of a wearable stimulator and wireless programmer. Positive top line data were reported in September 2022 from the company’s first pivotal study, called Up-LIFT, evaluating the ability of ARC-EX Therapy to improve upper extremity strength and function. The company is now preparing marketing approval submissions for the U.S. and Europe. ARC-IM consists of an implantable pulse generator and lead that is placed near the spinal cord. The company completed its first-in-human use of the ARC-IM neurostimulator in May 2022.
ONWARD is headquartered at the High Tech Campus in Eindhoven, the Netherlands. It has substantial operations in Lausanne, Switzerland, and a growing U.S. presence in Boston, Massachusetts. For additional information about the company, please visit ONWD.com. To access our 2022 Financial Calendar, please visit IR.ONWD.com.
For Company Enquiries:
For Media Enquiries:
MC Services AG
US: Laurie Doyle, P: +1 339 832 0752
Europe: Dr. Johanna Kobler, Katja Arnold, Kaja Skorka P: +49 89 210 228 0,
For Investor Enquiries:
Disclaimer
Certain statements, beliefs, and opinions in this press release are forward-looking, which reflect the Company or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve several risks, uncertainties, and assumptions that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, changes in demand, competition, and technology, can cause actual events, performance, or results to differ significantly from any anticipated development. Forward-looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions, or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.
Sesen Bio and Carisma Therapeutics Announce Merger Agreement
- Transaction to create a well-funded, clinical-stage biotechnology company advancing engineered macrophages for the treatment of cancer and other serious disorders
- Combined company expected to have approximately $180 million of cash, cash equivalents and marketable securities at close, including $30 million from a concurrent financing by Carisma, which is expected to fund the combined company through 2024
- Cash runway of combined company expected to enable multiple clinical readouts across Carisma programs
CAMBRIDGE, Mass. and PHILADELPHIA, Penn., Sep. 21, 2022 – Sesen Bio, Inc. (Nasdaq: SESN) and Carisma Therapeutics Inc. (Carisma), a privately held, clinical stage biopharmaceutical company focused on discovering and developing innovative immunotherapies, announced today that they have entered into a definitive merger agreement to combine the companies in an all-stock transaction. The combined company will focus on the advancement of Carisma’s proprietary cell therapy platform that utilizes engineered macrophages and monocytes to potentially transform the treatment of cancer and other serious disorders. Carisma is pioneering the development of chimeric antigen receptor macrophage (CAR-M) therapies and is believed to be the only company developing CAR-M therapies with demonstrated proof of mechanism and safety data in clinical trials. The combined company is expected to operate under the name Carisma Therapeutics Inc. and trade on Nasdaq under the ticker symbol “CARM”.
Carisma has also secured commitments from a syndicate of investors for a $30 million financing, including HealthCap, AbbVie, Wellington Partners, SymBiosis, Penn Medicine, TPG Biotech, MRL Ventures Fund, the therapeutics-focused corporate venture arm of Merck & Co., Agent Capital, Solasta, Livzon, Pictet Alternative Advisors and 4Bio, which is expected to close concurrently with the completion of the merger. With the cash expected from both companies at closing and the proceeds of the concurrent financing, the combined company is expected to have approximately $180 million in cash, cash equivalents and marketable securities. These cash resources are expected to be used to advance Carisma’s pipeline through multiple ongoing and planned key data readouts across several clinical trials and to fund operating expenses and capital expenditure requirements through 2024. The merger and related financing are expected to close in the next three to four months.
Carisma’s cell and gene therapies are based on a proprietary platform technology that reprograms a patient’s macrophages and targets them against cancer cells, with the potential for broad anti-tumor immunity. This novel technology is designed to engage with the body’s immune system to treat solid tumors, which remains a persistent clinical challenge that is yet to be comprehensively achieved through CAR-T and other immunotherapy approaches.
Carisma’s CAR-M platform provides the ability to fine-tune the specific targets of the immune cells, potentially enabling multiple therapeutic applications in and beyond oncology. The first clinical application of this technology is CT-0508, a CAR-M cell therapy currently being evaluated by Carisma in a Phase 1 multi-center clinical trial with a lead target indication of advanced HER2+ solid tumors. Carisma believes this Phase 1 clinical trial marks the first time that engineered macrophages are being studied in humans. Carisma is leveraging its proprietary CAR-M platform to expand its oncology pipeline both independently and through a strategic partnership with Moderna. Additionally, Carisma is exploring the potential to develop its proprietary macrophage engineering platform for non-oncology applications such as liver fibrosis, as well as autoimmune and neurodegenerative disease indications.
“The proposed merger represents an exciting opportunity for shareholders of each company, and we believe it gets us one step closer to our goal of revolutionizing the field of immunotherapy,” said Steven Kelly, President and Chief Executive Officer of Carisma. “This transaction will provide us with financial strength to not only continue to develop our lead candidate CT-0508, but also allow us to accelerate the growth of our platform and pipeline within and outside of oncology and develop additional strong strategic partnerships beyond those we already have with Moderna and Novartis. Carisma is focused on delivering cutting-edge technology for patients in a way that has never been done before, and we look forward to advancing this important mission.”
“This transaction represents the result of a thoughtful and careful review of strategic alternatives over the past four months, during which Carisma’s clinical programs, management team, and corporate strategy stood out amongst the 42 bids reviewed,” said Dr. Thomas Cannell, President and Chief Executive Officer of Sesen Bio. “Carisma is an exciting clinical-stage company with groundbreaking science and an impressive management team, which we believe makes them the optimal partner to provide value for our shareholders. Our mission at Sesen Bio has always been to save and improve the lives of patients with cancer, and we believe Carisma has the science and the unwavering patient focus required to make that mission a reality.”
Carisma has several anticipated upcoming catalysts and developmental milestones across its clinical programs over the next 18 months, including:
- Additional Phase 1 data readout of safety, manufacturing feasibility, and mechanism of action of CT-0508 with single-day dosing
- Completion of the technology transfer to Novartis for the planned clinical manufacturing of CT-0508
- Phase 1 data readout for the CT-0508 intraperitoneal trial for patients with HER2+ peritoneal cancer
- Phase 1 data readout of CT-0508 in combination with pembrolizumab for patients with HER2+ solid tumors
- Investigational New Drug (IND) Application for a new HER2 CAR engineered monocyte cell product
In addition to its exclusively licensed proprietary technologies that were developed by leading scientists at the University of Pennsylvania (Penn), including Saar Gill, MD, PhD, an associate professor of Medicine at Penn’s Perelman School of Medicine and a Carisma co-founder and fellow co-founder and Carisma’s Chief Scientific Officer, Dr. Michael Klichinsky, PharmD, PhD, Carisma has well-established strategic partners to support the advancement of its pipeline. Carisma recently entered into a strategic partnership with Moderna for the discovery, development and commercialization of in vivo CAR-M therapies for up to 12 targets for the treatment of cancer. As part of the collaboration, Carisma received a $45 million up-front cash payment and an investment by Moderna in the form of a $35 million convertible note, which will convert into shares of common stock of the combined company in connection with the merger. Under the collaboration, Carisma will receive full research funding and is eligible to receive development, regulatory, and commercial milestone payments, plus royalties on net sales of any products that are commercialized. Carisma has also partnered with Novartis, which has extensive experience in cell therapy manufacturing, to operate as Carisma’s contract manufacturing organization for clinical supply of its lead clinical program, CT-0508.
About the Proposed Merger
Pre-merger Sesen Bio stockholders are expected to own approximately 41.7% and pre-merger Carisma stockholders are expected to own approximately 58.3% of the combined company, in each case before giving effect to the concurrent financing described above and the conversion of the outstanding Moderna convertible note. Under the terms of the merger agreement, stockholders of Carisma will receive newly issued shares of Sesen Bio common stock pursuant to an exchange ratio formula set forth in the merger agreement. The percentage of the combined company that Sesen Bio stockholders will own upon the closing of the merger is further subject to adjustment based on the amount of Sesen Bio’s net cash at the time of closing.
Immediately prior to the closing of the proposed merger, Sesen Bio stockholders of record will be issued a contingent value right (CVR) for each outstanding share of Sesen Bio common stock held by such Sesen Bio stockholder as of such date, representing the right to receive certain cash payments from proceeds received by Sesen Bio related to the Roche Asset Purchase Agreement, if any, subject to customary deductions, including for expenses and taxes.
Following the consummation of the merger, the combined company will be headquartered in Philadelphia, Pennsylvania, and will be led by Steven Kelly, President and Chief Executive Officer of Carisma. Mr. Kelly was recently named Ernst & Young Entrepreneur of the Year 2022 Greater Philadelphia, an award that recognizes the most ambitious leaders who are building and sustaining successful, dynamic businesses around the world. The board of directors of the combined company is expected to be composed of seven members, consisting of one member designated by Sesen Bio and six members designated by Carisma.
The merger agreement has been unanimously approved by the boards of directors of both companies. The merger and related financing are expected to close in the next three to four months, subject to approval by Sesen Bio’s shareholders and other customary closing conditions.
Additional information about the transaction will be provided in a Current Report on Form 8-K that will be filed by Sesen Bio with the Securities and Exchange Commission (SEC) and will be available at www.sec.gov.
SVB Securities is acting as exclusive financial advisor to Sesen Bio for the transaction and Hogan Lovells US LLP is serving as its legal counsel. Evercore Group LLC is serving as lead financial advisor to Carisma for the transaction and BofA Securities, Inc. is also serving as financial advisor to Carisma for the transaction. Wilmer Cutler Pickering Hale and Dorr LLP is serving as legal counsel to Carisma. BofA Securities, Inc. and Evercore Group L.L.C. are serving as co-placement agents for Carisma’s concurrent financing and Shearman & Sterling LLP is serving as the placement agents’ legal counsel.
About Sesen Bio
Sesen Bio, Inc. is a late-stage clinical company focused on targeted fusion protein therapeutics for the treatment of patients with cancer. Sesen Bio’s most advanced product candidate, Vicineum™, also known as VB4-845, is a locally-administered targeted fusion protein composed of an anti-epithelial cell adhesion molecule antibody fragment tethered to a truncated form of Pseudomonas exotoxin A for the treatment of non-muscle invasive bladder cancer. On July 15, 2022, Sesen Bio made the strategic decision to voluntarily pause further development of Vicineum in the US. The decision was based on a thorough reassessment of Vicineum, which included the incremental development timeline and associated costs for an additional Phase 3 clinical trial, following Sesen Bio’s discussions with the United States Food and Drug Administration. Sesen Bio has turned its primary focus to assessing potential strategic alternatives with the goal of maximizing shareholder value. Additionally, Sesen Bio intends to seek a partner for the further development of Vicineum. For more information, please visit the Company’s website at www.sesenbio.com.
About Carisma Therapeutics
Carisma Therapeutics Inc. is a biopharmaceutical company dedicated to developing a differentiated and proprietary cell therapy platform focused on engineered macrophages, cells that play a crucial role in both the innate and adaptive immune response. The first applications of the platform, developed in collaboration with the University of Pennsylvania*, are autologous chimeric antigen receptor (CAR)-macrophages for the treatment of solid tumors. Carisma Therapeutics is headquartered in Philadelphia, PA. For more information, please visit www.carismatx.com
*Carisma has licensed certain Penn-owned intellectual property from the University of Pennsylvania, and Penn's Perelman School of Medicine receives sponsored research and clinical trial funding from Carisma. Penn and certain of its faculty members, including Dr. Gill, are current equity holders in Carisma and have received and may be entitled to receive future financial consideration from Carisma from the development and commercialization of products based on licensed Penn intellectual property.
Cautionary Note on Forward-Looking Statements
Any statements in this press release about future expectations, plans and prospects for Sesen Bio, Carisma or the combined company, Sesen Bio’s, Carisma’s or the combined company’s strategy or future operations, and other statements containing the words “anticipate,” “believe,” “contemplate,” “expect,” “intend,” “may,” “plan,” “predict,” “target,” “potential,” “possible,” “will,” “would,” “could,” “should,” “continue,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. For example, statements concerning the proposed transaction, the concurrent financing, the contingent value rights and other matters, including without limitation: statements relating to the satisfaction of the conditions to and consummation of the proposed transaction, the expected timing of the consummation of the proposed transaction and the expected ownership percentages of the combined company, Sesen Bio’s and Carisma’s respective businesses, the strategy of the combined company, future operations, advancement of the combined company’s product candidates and product pipeline, clinical development of the combined company’s product candidates, including expectations regarding timing of initiation and results of clinical trials of the combined company, the ability of Sesen Bio to remain listed on the Nasdaq Stock Market, the completion of the concurrent financing, and the receipt of any payments under the contingent value rights are forward-looking statements. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including without limitation: (i) the risk that the conditions to the closing of the proposed transaction are not satisfied, including the failure to obtain stockholder approval of matters related to the proposed transaction in a timely manner or at all; (ii) uncertainties as to the timing of the consummation of the proposed transaction and the ability of each of Sesen Bio and Carisma to consummate the proposed transaction, including completing the concurrent financing; (iii) risks related to Sesen Bio’s ability to correctly estimate its expected net cash at closing and Sesen Bio’s and Carisma’s ability to correctly estimate and manage their respective operating expenses and expenses associated with the proposed transaction; (iv) risks related to Sesen Bio’s continued listing on the Nasdaq Stock Market until closing of the proposed transaction; (v) the risk that as a result of adjustments to the exchange ratio, Sesen Bio stockholders or Carisma stockholders could own less of the combined company than is currently anticipated; (vi) the risk that the conditions to payment under the contingent value rights will not be met and that the contingent value rights may otherwise never deliver any value to Sesen Bio stockholders; (vii) risks associated with the possible failure to realize certain anticipated benefits of the proposed transaction, including with respect to future financial and operating results; (viii) uncertainties regarding the impact any delay in the closing would have on the anticipated cash resources of the combined company upon closing and other events and unanticipated spending and costs that could reduce the combined company’s cash resources; (ix) the occurrence of any event, change or other circumstance or condition that could give rise to the termination of the merger agreement; (x) the effect of the announcement, pendency or completion of the merger on Sesen Bio’s or Carisma’s business relationships, operating results and business generally; (xi) costs related to the merger; (xii) the outcome of any legal proceedings that may be instituted against Sesen Bio, Carisma or any of their respective directors or officers related to the merger agreement or the transactions contemplated thereby; (xiii) the ability of Sesen Bio or Carisma to protect their respective intellectual property rights; (xiv) competitive responses to the proposed transaction and changes in expected or existing competition; (xv) the success and timing of regulatory submissions and pre-clinical and clinical trials; (xvi) regulatory requirements or developments; (xvii) changes to clinical trial designs and regulatory pathways; (xviii) changes in capital resource requirements; (xix) risks related to the inability of the combined company to obtain sufficient additional capital to continue to advance its product candidates and its preclinical programs; (xx) legislative, regulatory, political and economic developments; and (xxi) other factors discussed in the “Risk Factors” section of Sesen Bio’s Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other reports filed with the SEC. In addition, the forward-looking statements included in this communication represent Sesen Bio’s and Carisma’s views as of the date hereof. Sesen Bio and Carisma anticipate that subsequent events and developments will cause the respective company’s views to change. However, while Sesen Bio may elect to update these forward-looking statements at some point in the future, Sesen Bio specifically disclaims any obligation to do so, except as required under applicable law. These forward-looking statements should not be relied upon as representing Sesen Bio’s views as of any date subsequent to the date hereof.
Important Additional Information
In connection with the proposed transaction, Sesen Bio will file materials with the SEC, including a registration statement on Form S-4 (Form S-4), which will include a document that serves as a proxy statement/prospectus of Sesen Bio and an information statement of Carisma, and other documents regarding the proposed transaction. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THESE MATERIALS, INCLUDING THE FORM S-4 AND THE PROXY STATEMENT/PROSPECTUS, WHEN THEY BECOME AVAILABLE, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION AND THE PARTIES TO THE PROPOSED TRANSACTION. Investors and security holders will be able to obtain the Form S-4, the proxy statement/prospectus and other materials filed by Sesen Bio with the SEC free of charge from the SEC’s website at www.sec.gov or from Sesen Bio at the SEC Filings section of www.sesenbio.com.
No Offer or Solicitation
This press release shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Subject to certain exceptions to be approved by the relevant regulators or certain facts to be ascertained, a public offer will not be made directly or indirectly, in or into any jurisdiction where to do so would constitute a violation of the laws of such jurisdiction, or by use of the mails or by any means or instrumentality (including without limitation, facsimile transmission, telephone or internet) of interstate or foreign commerce, or any facility of a national securities exchange, of any such jurisdiction.
Participants in the Solicitation
Sesen Bio and Carisma and their respective directors, executive officers and other members of management may be deemed to be participants in the solicitation of proxies in respect of the proposed transaction. Information about Sesen Bio’s directors and executive officers is available in Sesen Bio’s Annual Report on Form 10-K for the fiscal year ended December 31, 2021, its definitive proxy statement dated April 28, 2022 for its 2022 Annual Meeting of Stockholders and its Current Report on Form 8-K filed with the SEC on August 31, 2022. Other information regarding the participants in the proxy solicitation and a description of their interests in the transaction, by security holdings or otherwise, will be included in the proxy statement/prospectus and other relevant materials to be filed with the SEC regarding the proposed transaction when they become available. Investors should read the proxy statement/prospectus carefully when it becomes available before making any voting or investment decisions. You may obtain free copies of these documents from Sesen Bio or the SEC’s website as indicated above.
Investors:
Erin Clark, Vice President, Corporate Strategy & Investor Relations
Carisma Media Contact:
Julia Stern
(763) 350-5223
Aignostics Raises €14m Series A to Advance AI-powered Pathology
Aignostics Raises €14m Series A to Advance AI-powered Pathology
- Oversubscribed financing round with top-tier life science investors, led by Wellington Partners
- Spin-off from Charité – Universitätsmedizin Berlin developing sophisticated AI models to improve understanding, diagnosis and treatment of complex diseases
- Funds to expand US market presence and further advance AI platform
Berlin, Germany, 15th September 2022
Aignostics, a spin-off from Charité – Universitätsmedizin Berlin, developing novel digital pathology solutions with “Explainable AI” for pharmaceutical research and diagnostics, today announced the closing of an oversubscribed €14m Series A financing round. Wellington Partners led the round, joined by existing investors Boehringer Ingelheim Venture Fund (BIVF), VC Fonds Technologie managed by IBB Ventures and High-Tech Gründerfonds (HTGF). CARMA Fund, initiated by Ascenion, also participated as a new investor.
While pathologists are outstanding at interpreting individual tissue samples qualitatively, deep analysis of large data sets is a challenge better suited for AI. Recognizing that, Aignostics focuses on building leading AI models for the detailed analysis of tissue samples and associated metadata for its blue-chip pharma and biotech clients. Aignostics’ AI models go well beyond current off-the-shelf solutions and established approaches. Their models cover key tissue staining technologies including hematoxylin-eosin (H&E), immunohistochemistry (IHC) and multiplex immunofluorescence (mIF). They enable the acceleration of pre-clinical and translational research to improve understanding of disease biology, mode of action, or novel biomarkers and drug response characteristics, which are difficult to assess with traditional approaches under a microscope.
“With this new round of funding, we want to continue building our presence and team. In particular, we will put a stronger focus on the US market, in which we have been active since the beginning of this year,” explained Viktor Matyas, CEO and co-founder of Aignostics. “By accelerating our investments into our platform and regulatory excellence, we will be able to offer a Good Clinical Practice (GCP) compliant development and analysis process starting early 2023, and we plan to have first AI models in use in early-stage clinical trials soon thereafter. Eventually, we aim to become a credible choice for global Companion Diagnostic (CDx) developments too.”
“In addition, we will continue driving innovation in machine learning research. For instance, our patented “Explainable AI” technology reverse engineers traditional black-box AI models and reveals features that its decisions and predictions are based on. This helps discover novel biomarkers or drug response characteristics. Moreover, we have developed a proprietary approach for the automated training of cell-level AI that does not rely on manual annotations by pathologists. Both approaches have successfully been deployed in client studies, and there are several more areas of research that we have identified as highly promising and impactful,” said Dr. Maximilian Alber, CTO and co-founder.
“What makes Aignostics unique is not just its technology, but also its comprehensive access to key data modalities and pathologists, who are closely involved in the development process at all times. This combination allows Aignostics to rapidly build bespoke pre-trained AI models that are highly impactful when used by its clients on their own research and clinical trial data,” said Dr. Johannes Fischer, Principal at Wellington Partners. The offering is “tissue centric”, meaning there is always a stained tissue sample at the core of the analysis, but often involving metadata and additional data modalities, such as molecular data or clinical data.
Dr. Alexander Ehlgen from Boehringer Ingelheim Venture Fund added: “We have been very pleased with the development of Aignostics over the last two years and believe the team has demonstrated the value it can add to its pharma and biotech clients. BIVF is therefore excited to continue supporting Aignostics’ growth and development, and in particular its research activities, as the Company builds out its market-leading technology and IP.”
Dr. Rainer Strohmenger, Managing Partner at Wellington Partners commented: “We are deeply impressed by the Aignostics team and technology. Wellington is excited to join the group of existing investors and to support a leading German AI player during its expansion phase. Even more than our previous portfolio company Definiens, Aignostics has the potential to transform how the pharmaceutical industry and CROs use pathology in their drug development process, with the goal to significantly advance treatments for patients with hard-to-treat diseases, including cancer.”
ABOUT AIGNOSTICS
Aignostics specializes in AI-powered pathology, uniquely combining proprietary technology, its pathologist network, and comprehensive access to key data modalities to build bespoke AI models for its global pharma and biotech client base. These AI models can deliver valuable insights into disease biology from tissue samples, such as novel biomarkers and drug response characteristics.
Aignostics formally started in 2018 in the Digital Health Accelerator program of the Berlin Institute of Health (BIH), based on joint research by Charité – Universitätsmedizin Berlin, one of Europe’s largest university hospitals, led by Prof. Frederick Klauschen, and TU Berlin, led by Prof. Klaus-Robert Müller. In early 2020, Aignostics was spun-out of Charité. To date, Aignostics has raised close to €20m in funding from leading VC investors and has offices in Berlin, Germany, and Boston, U.S.
Contact Media contact
Aignostics GmbH MC Services AG
Viktor Matyas, CEO Kaja Skorka, Dr. Regina Lutz
Phone: +49 89-210 2280
ABOUT WELLINGTON PARTNERS
Wellington Partners is a leading European venture capital firm investing in the most promising early- and growth stage life science companies in the fields of biotechnology, therapeutics, medical technology, diagnostics and digital health. With funds totalling more than €1.2 billion, thereof €590 million committed to life sciences, Wellington Partners has been actively supporting world class private companies translating true innovation into successful businesses with exceptional growth. To date, Wellington Partners has invested in 56 innovative life science companies, including Actelion (acquired by J&J), Definiens (acquired by AZ), immatics (Nasdaq: IMTX). invendo (acquired by Ambu), MTM Laboratories (acquired by Roche/Ventana), Oxford Immunotec (acquired by PerkinElmer), Rigontec (acquired by MSD), Symetis (acquired by Boston Scientific), and Themis (acquired by MSD).
ABOUT BOEHRINGER INGELHEIM VENTURE FUND GMBH
Created in 2010, the Boehringer Ingelheim Venture Fund GmbH (BIVF) invests in ground-breaking companies to drive innovation in biomedical research. BIVF is searching for significant enhancements in patient care through pioneering science and its clinical translation by building long-term relationships with scientists and entrepreneurs. BIVF’s focus is to target unprecedented concepts addressing high medical needs in immuno-oncology, regenerative medicine, infectious diseases and digital health. For more information, please visit:
www.boehringer-ingelheim-venture.com
ABOUT IBB VENTURES
IBB Ventures (www.ibbventures.de) has been providing venture capital to innovative Berlin-based companies since 1997 and has established itself as the market leader in early stage financing. The funds are primarily used for development and market launch of innovative products or services as well as for business concepts from creative industries. Two funds with a total volume of EUR 122 million are currently in the investment phase. Both VC funds are financed by the Investitionsbank Berlin (IBB) and the European Regional Development Fund (ERDF), managed by the State of Berlin. IBB Ventures has already invested in more than 260 creative and technology companies in Berlin; in syndicates with partners, the start-ups received approx. EUR 1.7 billion, of which IBB Ventures has invested EUR 250 million as lead, co-lead or co-investor.
ABOUT HIGH-TECH GRÜNDERFONDS
High-Tech Gründerfonds (HTGF) is a seed investor that finances high-potential, tech-driven start-ups. With around EUR 900 million in total investment volume across three funds and an international network of partners, HTGF has already helped forge more than 670 start-ups since 2005. With the start of HTGF IV, more than EUR 400 million in fund volume will be added in the fall of 2022. Driven by their expertise, entrepreneurial spirit and passion, its team of experienced investment managers and startup experts help guide the development of young companies. HTGF’s focus is on high-tech start-ups in the fields of digital tech, industrial technology, life sciences, chemistry and related business areas. To date, external investors have injected more than EUR 4 billion into the HTGF portfolio via more than 1,900 follow-on financing rounds. HTGF has also successfully sold interests in more than 160 companies.
Fund Investors in the public-private partnership include the Federal Ministry for Economic Affairs and Climate Action, KfW Capital, the Fraunhofer-Gesellschaft and many companies from a wide range of industries.
www.htgf.de
ABOUT CARMA FUND
CARMA FUND is an investment fund for the advancement of early-stage Life Science and Healthcare assets and companies. The fund is uniquely suited for young projects from the medical space with their extended development timelines due to its extra-long term and flexible investment modes. It started off with a First Closing of €47M in June 2022 and is based in Munich and Frankfurt.
ABOUT ASCENION GMBH
Ascenion is an independent technology transfer company focussing on the life sciences. It is partner to 30 research organizations, universities and university hospitals in Germany and Europe. Particular strengths are spin-off support and project development. In 2022 Ascenion initiated the CARMA FUND which invests in early-stage projects and start-ups in the life-science and healthcare sector. As technology transfer partner of the BIH and Charité Ascenion supported the founders and researchers in successfully implementing the spin-off from the BIH Digital Health Accelerator programme. In close cooperation with BIH, Ascenion also supported the negotiation of key agreements throughout the process and the series seed financing round.
ONWARD Reports Positive Topline Results from a Pivotal Study to Restore Arm and Hand Function in People with Spinal Cord Injury
ONWARD Reports Positive Topline Results from a Pivotal Study to Restore Arm and Hand Function in People with Spinal Cord Injury
THIS PRESS RELEASE CONTAINS INSIDE INFORMATION WITHIN THE MEANING OF ARTICLE 7(1) OF THE EUROPEAN MARKET ABUSE REGULATION (596/2014).
Up-LIFT study achieves primary endpoint: Statistically significant and clinically meaningful improvement in upper extremity strength and functioni i
Improvement in arm and hand function is the highest priority among people with tetraplegiaii ii
Up-LIFT is the first large-scale clinical study of non-invasive spinal cord stimulation technology
ONWARD plans to submit for marketing approval in the U.S. and Europe with the goal to launch ARC-EX Therapy in the second half of 2023
EINDHOVEN, the Netherlands, LAUSANNE, Switzerland, and BOSTON, MA USA—September 13, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury (SCI), today announced that the Up-LIFT pivotal study evaluating ARC-EX Therapy achieved its primary effectiveness endpoint of improvement in upper extremity strength and function. ARC-EX Therapy is a proprietary non-invasive spinal cord stimulation technology designed to restore movement and other functions in people with movement disabilities.
“The Up-LIFT study results represent a turning point in the field of spinal cord injury and paralysis science,” said Marco Baptista, Ph.D., Chief Scientific Officer of the Christopher & Dana Reeve Foundation. Functional recovery once deemed impossible may now be in reach. The Reeve Foundation looks forward to this technology advancing and, we hope, becoming widely available to our community.”
“Our vision is to empower people with spinal cord injury to enjoy life in every way that matters to them. Today’s excellent results from the Up-LIFT study will help us transform that vision into reality”, said Dave Marver, ONWARD CEO. “Our team is working hard to prepare regulatory submissions and toget ready for launch in the U.S. and Europe. We are hopeful we can begin to positively impact the lives of people with spinal cord injury sometime during the second half of 2023.”
“Restoring hand and arm function after spinal cord injury is life-changing, freeing people with paralysis to feed and care for themselves and be more independent in everyday activities,” said Chet Moritz, Ph.D., study co-Principal Investigator (co-PI) and Professor of Electrical & Computer Engineering and Rehabilitation Medicine at the University of Washington.
“We are grateful to the many therapists, clinicians, and people with SCI who participated in this landmark study. There was very low attrition over thousands of clinic visits, a testament to thecollective enthusiasm for this compelling therapy and for everyone’s determination to find new treatment options for people with SCI,” added Edelle Field-Fote, PT, Ph.D., FAPTA, FASIA, study co-PI, Director of SCI Research at the Shepherd Center in Atlanta, GA, and Professor at Emory University School of Medicine in the Department of Rehabilitation Medicine.
The Up-LIFT study is a prospective, single-arm pivotal study designed to evaluate the safety and effectiveness of non-invasive electrical spinal cord stimulation (ARC-EX Therapy) to treat upper extremity functional deficits in people with chronic tetraplegia (paralysis of all four limbs). The study enrolled 65 people at 14 leading SCI centers in the U.S., Europe, and Canada. Time since injury averaged 5.9 years (range 1 to 34 years) with an average subject age of 46.5 years. Detailed results will be made available after review by the FDA. The company plans to submit for regulatory approval in both the US and Europe within the next 6 months.
Participants completed an average of 50 training sessions over a period of about 4 months. A series of comprehensive assessments were performed at baseline and monthly thereafter to detect changes in sensory and motor function of upper extremities that directly translate into improved functional performance in activities of daily living. Rigorous measures such as CUE-T, GRASSP, ISNCSCI iii and pinch and grasp force were used to detect clinically meaningful changes resulting from the combination of ONWARD ARC-EX Therapy with a standard of care rehabilitation. An independent data safety monitoring board adjudicated the safe conduct of the study.
About Spinal Cord Injury
Spinal cord injury (SCI) represents a major unmet medical need for which there is no cure. Approximately 7 million people globally have a spinal cord injury, with over 650,000 in the U.S. and Europe alone. The quality of life of people with SCI can be poor, with paralysis and loss of sensation, issues with blood pressure control and trunk stability, increased potential for infection, incontinence, and loss of sexual function. Assistance is required for daily living activities. And SCI is costly, with the average lifetime cost for a paraplegic (paralysis of the legs) of $2.5 million and $5 million for a tetraplegic (paralysis of all four limbs). Treatments are urgently needed to restore movement and improve quality of life.
About ONWARD Medical
ONWARD is a medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injuries. ONWARD’s work builds on more than a decade of basic science and preclinical research conducted at the world’s leading neuroscience laboratories. ONWARD’s ARC Therapy, which can be delivered by implantable (ARC-IM) or external (ARC-EX) systems, is designed to deliver targeted, programmed spinal-cord stimulation to restore movement and other functions in people with spinal cord injury, ultimately improving their quality of life.
ONWARD has received three Breakthrough Device Designations from the U.S. FDA encompassing both ARC-IM and ARC-EX. ARC-EX is an external, non-invasive platform consisting of a wearable stimulator and wireless programmer. Topline data were reported in September 2022 from the company’s first pivotal study, called Up-LIFT, evaluating the ability of ARC-EX Therapy to improve upper extremity strength and function. The company is now preparing marketing approval submissions for the U.S. and Europe. ARC-IM consists of an implantable pulse generator and lead that is placed near the spinal cord. The company completed its first-in-human use of the ARC-IM neurostimulator in May 2022.
ONWARD is headquartered at the High Tech Campus in Eindhoven, the Netherlands. It maintains an office in Lausanne, Switzerland, and has a growing U.S. presence in Boston, Massachusetts, USA. For additional information about the company, please visit ONWD.com. To access our 2022
Financial Calendar, please visit IR.ONWD.com.
For Company Enquiries:
For Media Enquiries:
MC Services AG
US: Laurie Doyle, P: +1 339 832 0752
Europe: Dr. Johanna Kobler, Katja Arnold, Kaja Skorka P: +49 89 210 228 0
For Investor Enquiries:
Disclaimer
Certain statements, beliefs and opinions in this press release are forward-looking, which reflect the Company or, as appropriate, the Company directors’ current expectations and projections about future events. By their nature, forward-looking statements involve several risks, uncertainties, and assumptions that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties and assumptions could adversely affect the outcome and financial effects of the plans and events described herein. A multitude of factors including, but not limited to, changes in demand, competition, and technology, can cause actual events, performance, or results to differ significantly from any anticipated development. Forward-looking statements contained in this press release regarding past trends or activities should not be taken as a representation that such trends or activities will continue in the future. As a result, the Company expressly disclaims any obligation or undertaking to release any update or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions, or circumstances on which these forward-looking statements are based. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person’s officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.
i Results pending review by FDA
ii Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004
Oct;21(10):1371-83. doi: 10.1089/neu.2004.21.1371. PMID: 15672628.
iii ISNCSCI = International Standards for Neurological Classification of Spinal Cord Injury
GRASSP = The Graded Redefined Assessment of Strength, Sensation, and Prehension
CUE-T = The Capabilities of the Upper Extremity Test
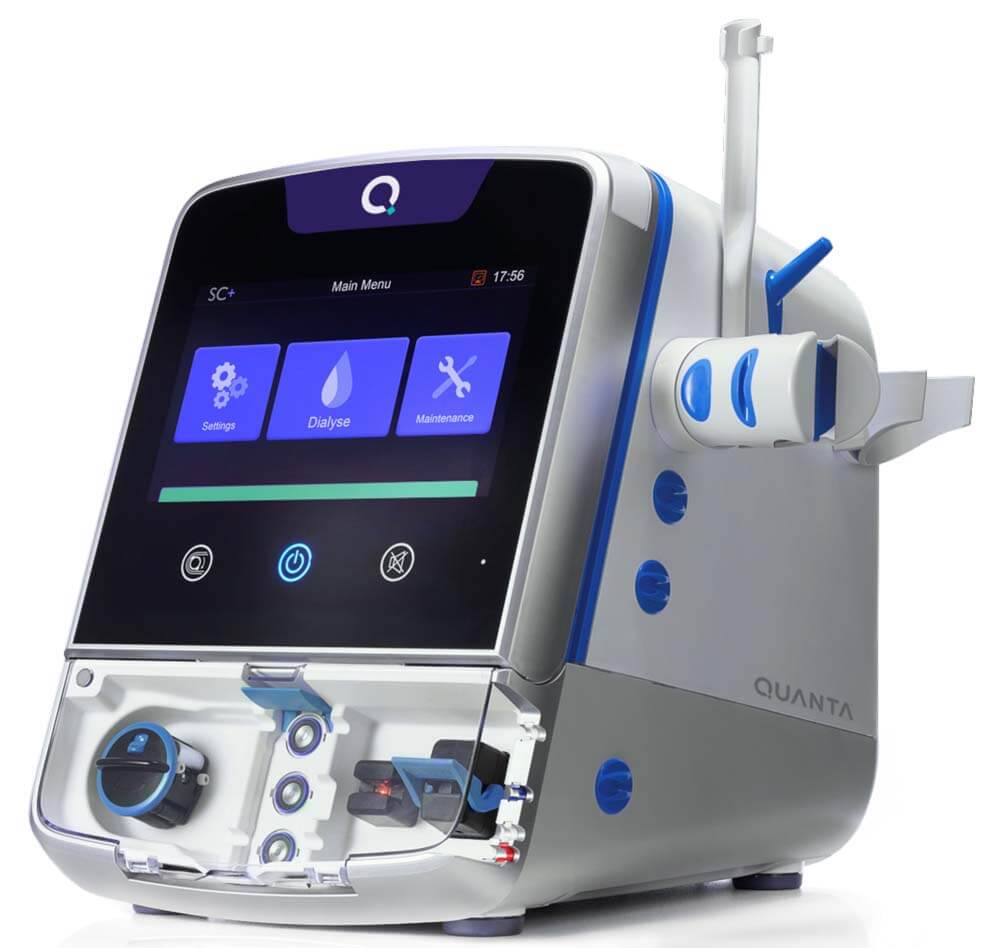
Quanta Dialysis Technologies: Team behind compact dialysis machine wins award for UK’s top engineering innovation of 2022
Quanta Dialysis Technologies: Team behind compact dialysis machine wins award for UK’s top engineering innovation of 2022
Quanta Dialysis Technologies presented with the 2022 MacRobert Award by HRH The Princess Royal
The team behind a revolutionary compact dialysis machine that enables kidney failure patients to treat themselves at home and relieves pressure on over-stretched hospitals has won the UK’s leading engineering innovation award for 2022.
The Royal Academy of Engineering has today announced Quanta Dialysis Technologies as the recipient of the 2022 MacRobert Award, the UK’s longest-running and most prestigious award for UK engineering innovation. Joining previous MacRobert Award winners including Rolls-Royce, Arup and Raspberry Pi, Quanta was recognised for developing the SC+ system, a portable, easy-to-use, high performance dialysis machine allowing greater flexibility across the care continuum – from hospital to home.
The winning team from Quanta Dialysis Technologies includes:
- John Milad, CEO
- Professor Clive Buckberry FREng, Chief Engineer and Technology Officer
- Keith Heyes, Systems Engineering Director & Original Inventor
- Mark Wallace, Lead Innovations Engineer
- David Spurling, Lead Architect (Mechanical Engineering)
- Maddy Warren, Strategic Dialysis Advisor and Community Engagement Consultant
The MacRobert Award celebrates engineering developments that demonstrate outstanding engineering innovation, commercial success and tangible social benefits, and the SC+ haemodialysis system impressed judges across all three criteria. It is already having a dramatic impact on patient quality of life since it is easier to operate, faster to train on and as powerful as traditional in-centre dialysis machines. This flexibility also enables patients to treat themselves at home overnight, receiving more dialysis care than they would in clinical settings and eliminating the gap where patients go without dialysis over a weekend.
Quanta is already working with NHS Trusts and during lockdown provided its entire UK SC+ system stock to the NHS in order to relieve pressure on hospitals and ICUs.
Judges were also impressed by the enormous commercial potential. The SC+ is already FDA cleared and selling in the US, where the dialysis market is expected to exceed $12bn. In 2021, Quanta raised $245million to fund the rollout of the SC+ system in the US — the largest-ever private funding round for a dialysis device company.
The SC+ system marks a major advance in dialysis technology, which has seen little innovation in decades. It operates using a proprietary single-use cartridge that eliminates the need for expensive and time-consuming disinfection between treatments. Each cartridge incorporates a series of pneumatic membrane pumps, rather than the piston-driven pumps found in traditional dialysis machines. This provides highly accurate fluid management and enhanced distribution within the dialyser itself, which acts as an artificial kidney, while minimising cross contamination and bio-burden (the number of microorganisms living on a non-sterilised surface) between treatments.
Professor Sir Richard Friend FREng FRS, Chair of the Royal Academy of Engineering MacRobert Award judging panel, said:
“This UK-based global healthtech success story, which traces back to technology first developed for use in fruit juice dispensers, demonstrates remarkable engineering ingenuity. Recent success comes on the back of Quanta’s considerable journey as a company. The team has been working for a decade to develop a machine that dramatically improves patient care and quality of life, relieves pressure on hospitals and showcases the enormous commercial potential that cutting edge engineering can unlock. The team exemplifies the persistence, innovation and unconventional thinking that has long been a hallmark of the UK’s greatest engineering success stories and they are worthy winners of the MacRobert Award.”
John E Milad, CEO of Quanta Dialysis Technologies, said:
“I am incredibly proud of our team for developing an innovation deemed worthy of the prestigious MacRobert Award. This is truly an honour to all of us at Quanta.”
The Quanta team were announced as the winners at the Royal Academy of Engineering’s Awards Dinner on Tuesday 12 July at Leicester Square’s stunning new sustainably designed and engineered hotel, The Londoner. The team received a £50,000 prize and join an impressive line-up of previous MacRobert Award winners. The first award in 1969 was won jointly by Rolls-Royce for the Pegasus engine used in the iconic Harrier jump jet, and Freeman, Fox and Partners for designing the Severn Bridge. More recently, 2008 winner Touch Bionics i-Limb Hand has helped to transform medical prosthetics, while people across all seven continents still rely on winning innovations from the likes of Jaguar Land Rover, Raspberry Pi and Inmarsat.
Notes to editors:
First presented in 1969, the MacRobert Award is widely regarded as the most coveted in the industry, honouring the winning organisation with a gold medal and the team members with a cash prize of £50,000. Founded by the MacRobert Trust, the award is presented and run by the Royal Academy of Engineering, with support from the Worshipful Company of Engineers. The 2022 judging panel is made up of:
- Professor Sir Richard Friend FREng FRS (Chair of Judges), former Cavendish Professor of Physics, University of Cambridge; Founder, Cambridge Display Technology
- Naomi Climer CBE FREng, Chair of Council, International Broadcasting Convention (IBC); Former President Media Cloud Services, Sony
- Dr Andy Harter CBE DL FREng, Chair, Cambridge Network; Founder RealVNC
- Professor John Fisher CBE FREng FMedSci, former Professor of Mechanical Engineering, University of Leeds
- Professor Dame Julia King, The Baroness Brown of Cambridge DBE FREng FRS, Chair, The Carbon Trust
- Professor Gordon Masterton DL OBE FREng FRSE, Trustee; The MacRobert Trust; Chair of Future Infrastructure, University of Edinburgh; Former Vice-President, Jacobs
- Dr Ruth McKernan CBE FMedSci, Venture Partner, SV Health Investors & Dementia Discovery Fund
- Professor Phil Nelson CBE FREng, Professor of Acoustics, University of Southampton
- Dr Liane Smith CBE FREng, Director, Larkton Ltd; former SVP Digital Solutions, Wood Group
The Royal Academy of Engineering is harnessing the power of engineering to build a sustainable society and an inclusive economy that works for everyone. In collaboration with its Fellows and partners, it’s growing talent and developing skills for the future, driving innovation and building global partnerships, and influencing policy and engaging the public to tackle the greatest challenges of our age.
Annual Awards Dinner 2022. This year’s Royal Academy of Engineering Awards Dinner took place in London on Tuesday 12 July. Along with the announcement of the winner of this year’s MacRobert Award, the event will also celebrate the winners of other awards and prizes including the Major Project Award, The Princess Royal Silver Medals, the President’s Medal, the Rooke Award and the RAEng Engineers Trust Young Engineer of the Year. The headline sponsor of this year’s Awards Dinner is BAE Systems, with gold sponsors bp and Rolls-Royce.
ImCheck Closes Upsized EUR 96 Million (USD 103 Million) Financing to Advance Clinical Program of First-in-human Gamma-delta T Cell Activating Antibody and Accelerate Development of Disruptive Immunotherapeutic Pipeline
ImCheck Closes Upsized EUR 96 Million (USD 103 Million) Financing to Advance Clinical Program of First-in-human Gamma-delta T Cell Activating Antibody and Accelerate Development of Disruptive Immunotherapeutic Pipeline
- Significant round will enable the Company to advance ICT01, its lead anti-butyrophilin 3A antibody, to completion of randomized Phase II trials
- Series C adds global investors Earlybird, Andera Partners, Invus and patient organization The Leukemia & Lymphoma Society Therapy Acceleration Program® to the syndicate
Marseille, France, June 13, 2022 – ImCheck Therapeutics announced today the close of a EUR 96 million (USD 103 million) financing, co-led by Earlybird and Andera Partners. The Series C round (EUR 80 Million – USD 86 Million) and the last outstanding tranche of Series B converted in Series C shares (EUR 16 Million – USD 17.2 Million) solidifies ImCheck’s financial position and leadership in the gamma-delta T cell space. Invus and The Leukemia & Lymphoma Society Therapy Acceleration Program® also joined the round as new investors. Existing investors including the Growth Opportunity Fund of founding investor Kurma Partners, Eurazeo, Gimv, EQT Life Sciences (previously LSP), Boehringer Ingelheim Venture Fund, Pfizer Ventures, Bpifrance through its Innobio 2 and Large Venture funds, Wellington Partners, Agent Capital, Pureos Bioventures and Alexandria Venture Investments participated.
The proceeds will be used to support the Phase IIa expansion arms of EVICTION in solid tumors and hematologic cancers, and completion of randomized, double-blind, placebo-controlled clinical trials evaluating ImCheck’s lead candidate ICT01 in combination with a PD-1 inhibitor for multiple solid tumors. The Company also will apply the capital to investigate ICT01 in combination with other therapeutic agents, including IL-2, in the forthcoming EVICTION-2 clinical trial. The funding will accelerate the further advance toward the clinic of additional antibody candidates in immuno-oncology, auto-immune and infectious diseases. In addition, it will allow the Company to achieve pivotal study readiness for ICT01 and expand its footprint through extended clinical operations and regulatory affairs in Europe and the US.
“Since its inception, ImCheck has gained the support of a syndicate of outstanding international funds. In a highly challenging funding market, we have secured a significant fundraising through the addition of highly strategic and valuable investors from the U.S. and Europe, putting us in a position to further deliver on the immense promise of our pipeline,” stated Pierre d’Epenoux, Chief Executive Officer of ImCheck Therapeutics. “We view our singular proprietary position with butyrophilins, which offer powerful immunomodulation of both the innate and adaptive immune systems, as the key to therapeutic intervention for many disease indications and we value the support we are now gaining from The Leukemia & Lymphoma Society Therapy Acceleration Program® as a first investment from a cancer patient nonprofit organization.”
In conjunction with the financing round, Florent Gros (Earlybird) and Raphaël Wisniewski (Andera Partners) will join the Company’s Board of Directors.
Florent Gros, Partner at Earlybird, commented,“ImCheck’s approach to immuno-oncology is highly differentiated through the clinical demonstration of gamma-delta T cell activation, an area of immunology that has huge potential and interest from the biopharmaceutical community. At Earlybird, we support companies that dare to think differently and ImCheck’s innovative concept for immunomodulation could be a game-changer for a range of indications.”
Raphaël Wisniewski, Partner at Andera Partners, said, “Immune checkpoint inhibitors have heralded a new era in cancer treatments and ImCheck Therapeutics is pioneering the next generation of these immunotherapeutics. In watching their progress to date, we have seen the leadership team execute on a compelling vision for a butyrophilin-based therapeutic approach from the early development stage into a highly valuable clinical development program. We at Andera Partners are confident the company will move its groundbreaking technology forward to meet patients’ needs in a range of cancer indications with wider potential for auto-immune and infectious diseases.”
ImCheck Therapeutics’ immunotherapeutic technology is capable of overcoming the tumor’s resistance to adaptive immune responses through a novel superfamily of immune checkpoint targets, butyrophilins (BTNs). BTNs can be modulated to harness a wide range of immune cells including gamma-delta T cells, CD3, CD8, NK cells and macrophages, bridging the innate and adaptive immune responses. The company’s broad pipeline is built upon immunomodulation via BTN-targeting antibodies aimed either at activating the immune system in disease indications such as cancer or infectious diseases, or down-regulating immunity in auto-immune disorders.
Hans Henrik Christensen, Chief Financial Officer of ImCheck Therapeutics, added, “ImCheck has raised a total of EUR 154 million since inception. We truly appreciate the support from existing and new investors, which extends our cash runway until 2026. This enables us to further explore the ‘pipeline in a product’ opportunity we have with our lead clinical candidate, ICT01.”
Legal counsel for the Series C transaction provided by Dentons Europe and McDermott Will & Emery. Investor relations support provided by Trophic Communications. French media and communications support provided by ATCG Partners.
Press contacts:
US and EU:
Trophic Communications
Gretchen Schweitzer
+49 (0) 172 861 8540
France:
ATCG PARTNERS
Céline Voisin
+33 (0)9 81 87 46 72 / +33 (0)6 62 12 53 39
imcheck@atcg–partners.com
Immatics and Bristol Myers Squibb Expand Strategic Alliance to Develop Gamma Delta Allogeneic Cell Therapy Programs
Immatics and Bristol Myers Squibb Expand Strategic Alliance to Develop Gamma Delta Allogeneic Cell Therapy Programs
- New multi-program collaboration to develop allogeneic TCR-T/CAR-T programs brings together Immatics’ allogeneic gamma delta T cell therapy platform ACTallo® with Bristol Myers Squibb’s technologies and oncology drug development expertise
- Immatics to receive upfront payment of $60 million and additional milestone payments of up to $700 million per program plus tiered royalty payments of up to low double-digit percentages on net product sales across multiple programs under the new collaboration
- Per 2019 agreement, Bristol Myers Squibb to also add one additional autologous TCR-T target where Immatics will receive an upfront payment of $20 million and be eligible for milestone payments and royalties
Tuebingen, Germany, Houston & New York – June 2, 2022 – Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, and Bristol Myers Squibb (NYSE: BMY), today announced that they have expanded their strategic alliance to pursue the development of multiple allogeneic off-the-shelf TCR-T and/or CAR-T programs.
Under this collaboration, Bristol Myers Squibb and Immatics will develop two programs owned by Bristol Myers Squibb and both companies have an option to develop up to four additional programs each. The programs will utilize Immatics’ proprietary gamma delta T cell-derived, allogeneic Adoptive Cell Therapy (ACT) platform, called ACTallo®, and a suite of next-generation technologies developed by Bristol Myers Squibb.
Under the terms of this agreement, Immatics will receive an upfront payment of $60 million as well as up to $700 million per Bristol Myers Squibb program through development, regulatory and commercial milestone payments and tiered royalty payments of up to low double-digit percentages on net product sales. Immatics will be responsible for preclinical development of the initial two Bristol Myers Squibb-owned programs and will receive additional payment for certain activities that Immatics could perform at Bristol Myers Squibb’s request. Bristol Myers Squibb will assume responsibility for clinical development and commercialization activities of all Bristol Myers Squibb-owned programs thereafter.
In addition, Bristol Myers Squibb and Immatics will expand their 2019 collaboration agreement focused on autologous T cell receptor-based therapy (TCR-T), with the inclusion of one additional TCR target discovered by Immatics. As part of this expansion, Immatics will receive an upfront payment of $20 million and be eligible for milestone payments and royalties.
“The expansion of our collaboration with Bristol Myers Squibb significantly advances our allogeneic cell therapy development strategy,” commented Harpreet Singh, Ph.D., Chief Executive Officer and Co-Founder of Immatics. “We welcome opening another chapter of our work with a trusted partner and the expertise and capabilities both companies provide in cell therapy development to create novel medicines for cancer patients.”
“Today’s announcement represents an important part of our continued investment in next generation cell therapies that have the potential to provide transformative outcomes to patients with cancer,” said Rupert Vessey, M.A., B.M., B.Ch., FRCP, D.Phil., Executive Vice President, Research & Early Development, Bristol Myers Squibb. “We are excited to expand our collaboration with Immatics that allows us to combine their novel off-the-shelf platforms with our industry-leading research and manufacturing expertise in cell therapy to develop new allogeneic cell therapy treatments to potentially help patients with solid tumor malignancies.”
About ACTallo®
ACTallo® is Immatics’ proprietary allogeneic, off-the-shelf adoptive cell therapy platform based on gamma delta T cells sourced from healthy donors. Our manufacturing process is designed to create hundreds of doses from one single donor leukapheresis. Gamma delta T cells are abundant in the peripheral blood, show intrinsic anti-tumor activity, naturally infiltrate solid tumors and do not cause graft-vs-host disease – characteristics that make this cell type well suited for an allogeneic approach. The ACTallo® process engineers gamma delta T cells with chimeric antigen receptors (CARs) or T cell receptors (TCRs), thus accessing cancer cell surface targets as well as intracellular proteins that are presented as peptides on the surface of the cancer cell. This enables the redirection of gamma delta T cells to cancer cell targets. ACTallo® products will be available for patient treatment without the requirement for personalized manufacturing. Since these T cells originate from healthy individuals, they are not reliant on the potentially encumbered immune system of the cancer patient.
– END –
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information about Bristol Myers Squibb, visit us at BMS.com or follow us on LinkedIn, Twitter, YouTube, Facebook, and Instagram.
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
For regular updates about Immatics, visit www.immatics.com. You can also follow us on Twitter, LinkedIn and Instagram.
Bristol Myers Squibb Cautionary Statement Regarding Forward-Looking Statements:
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 regarding, among other things, the research, development and commercialization of pharmaceutical products and the agreement. All statements that are not statements of historical facts are, or may be deemed to be, forward-looking statements. Such forward-looking statements are based on current expectations and projections about our future financial results, goals, plans and objectives and involve inherent risks, assumptions and uncertainties, including internal or external factors that could delay, divert or change any of them in the next several years, that are difficult to predict, may be beyond our control and could cause our future financial results, goals, plans and objectives to differ materially from those expressed in, or implied by, the statements. These risks, assumptions, uncertainties and other factors include, among others, that the expected benefits of, and opportunities related to, the agreement may not be realized by Bristol Myers Squibb or may take longer to realize than anticipated, that Bristol Myers Squibb may fail to discover and develop any commercially successful allogeneic off-the-shelf TCR-T and/or CAR-T program product candidates through the agreement, that such product candidates may not receive regulatory approval for the indications described in this release in the currently anticipated timeline or at all, and if approved, whether such product candidates for such indications described in this release will be commercially successful. No forward-looking statement can be guaranteed. Forward-looking statements in this press release should be evaluated together with the many risks and uncertainties that affect Bristol Myers Squibb’s business and market, particularly those identified in the cautionary statement and risk factors discussion in Bristol Myers Squibb’s Annual Report on Form 10-K for the year ended December 31, 2021, as updated by our subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the Securities and Exchange Commission. The forward-looking statements included in this document are made only as of the date of this document and except as otherwise required by applicable law, Bristol Myers Squibb undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events, changed circumstances or otherwise.
Immatics Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the timing of product candidates and Immatics’ focus on partnerships to advance its strategy are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements.
For more information, please contact:
Immatics
| Media: |
| Anja Heuer, +49 89 540415-606, |
| Investors |
| Jordan Silverstein, +1 281-810-7545, |