TRiCares Announces Successful Implantation of Minimally Invasive Topaz Tricuspid Heart Valve Replacement System in Canada
TRiCares Announces Successful Implantation of Minimally Invasive Topaz Tricuspid Heart Valve Replacement System in Canada
TRiCares Announces Successful Implantation of Minimally Invasive Topaz Tricuspid Heart Valve Replacement System in Canada
Paris, France and Munich, Germany, May 16, 2022 – TRiCares SAS (“TRiCares”) a privately held pioneer in the field of minimally invasive treatment of tricuspid regurgitation, today is pleased to announce the successful implantation of its Topaz transfemoral tricuspid heart valve replacement system (“Topaz”) in Canada, with full elimination of tricuspid regurgitation confirmed at 30-day follow-up examination.
Heart valve diseases are among the most serious cardiac conditions, affecting more than 12.7 million patients in Europe and many more worldwide. In the last decade minimally invasive catheter-based solutions have been developed for other heart valve diseases, but none have been designed specifically for the tricuspid valve.
Tricuspid regurgitation is a frequent and serious disease for which open heart surgery and symptomatic pharmacologic treatment are the current standard treatment options. Owing to high mortality risk, access to open heart surgery is severely restricted and is not considered an option for more than 99% of patients with tricuspid regurgitation. The prognosis for patients without surgical repair is poor, with 2.2 years median survival. As such, there is an urgent need for minimally invasive, lower risk solutions to improve outcomes for patients with no other viable treatment options.
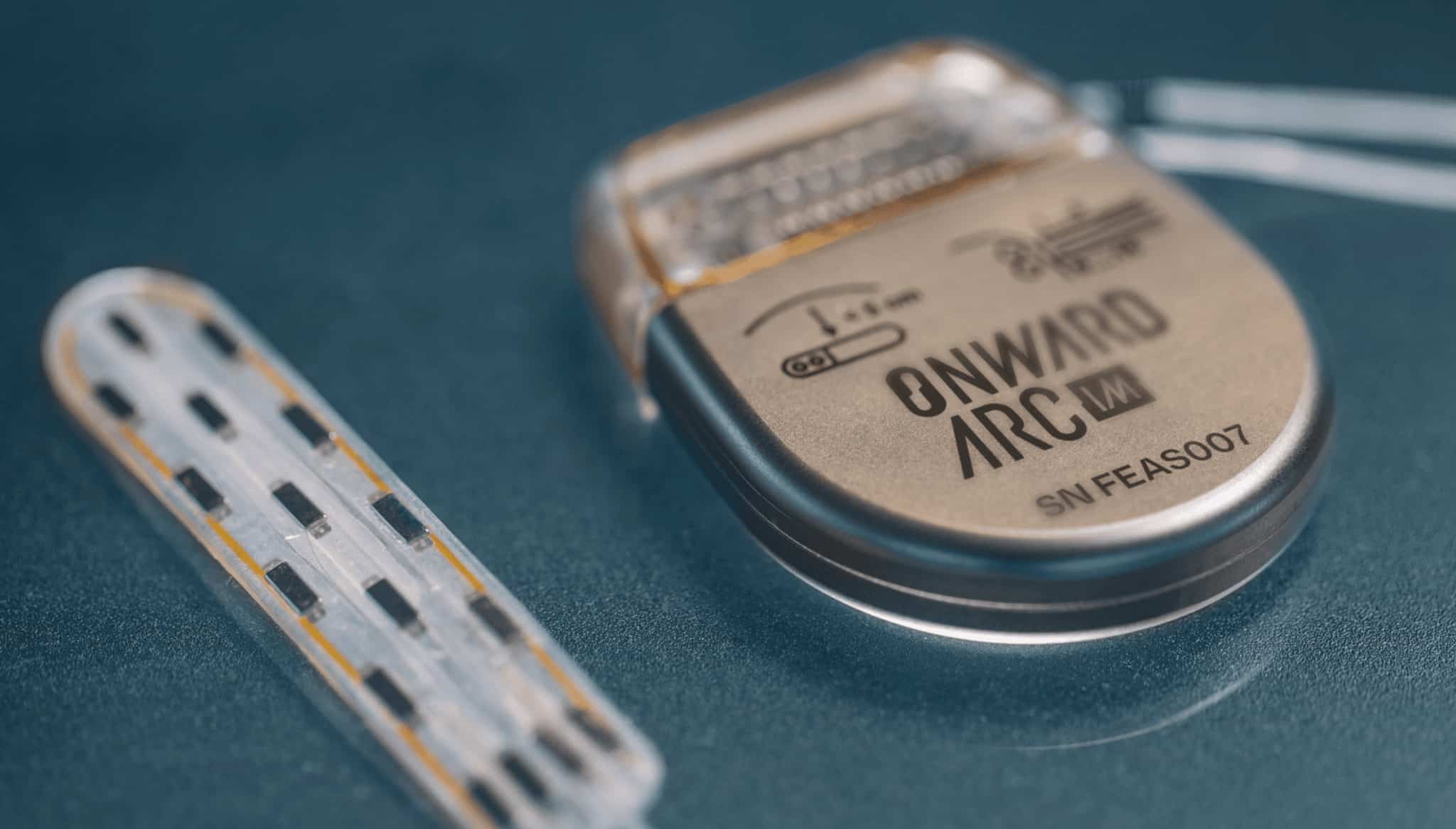
Topaz is an innovative device designed specifically to help patients suffering from severe tricuspid regurgitation without the need for open heart surgery. The Topaz device is the result of a French and German collaboration and is implanted in a minimally invasive procedure through the patient’s femoral vein. It is designed specifically to fit the tricuspid valve anatomy and thus supports ease of positioning and functionality.
Today’s announcement marks the successful first in human implantation of Topaz in a patient in Canada, which was performed after special access was granted by Health Canada.
The procedure in Canada was performed for a 50-year-old woman presenting with torrential tricuspid regurgitation, who was classed as having New York Heart Association (NYHA) class III heart failure. The patient has a history of two open heart valve surgeries as well as chemotherapy and radiation due to a sarcoma in the left atrium. For these reasons, transcatheter intervention was chosen, as the risk for the patient is lower compared to more invasive surgical intervention.
The successful implantation of the Topaz tricuspid heart valve replacement system took place at St. Michael’s Hospital, University of Toronto, on 12 April 2022, and was performed by Neil Fam, MD, MSc, Director of Interventional Cardiology and Cardiac Cath Labs at St. Michael’s Hospital, and assisted by cardiac surgeon Gianluigi Bisleri, MD, and Geraldine Ong, MD, MSc, a cardiologist specializing in echocardiography. Prof. Hendrik Treede, cardiac surgeon at University Hospital Mainz, Germany, proctored the procedure. With an implantation time of 20 minutes the Topaz prosthesis anchored safely and achieved complete correction of the tricuspid regurgitation. The patient recovered quickly from the intervention and was discharged from hospital after three days.
An examination of the patient 30 days after the procedure, including echo assessment, confirmed that the tricuspid regurgitation remains completely eliminated. The patient is now assessed as New York Heart Association (NYHA) class I, meaning no symptoms of heart failure and no limitations in ordinary physical activity.
In total nine implantations of the Topaz tricuspid heart valve replacement system have been performed to date, with the first implantation conducted almost one year ago.
Building upon the success of these procedures, TRiCares is preparing a clinical study in the coming months to confirm the value of its Topaz tricuspid heart valve replacement system for these types of patients, who until now have had no satisfactory treatment option.
Dr. Neil Fam, Director of Interventional Cardiology and Cardiac Cath Labs at St. Michael’s Hospital, University of Toronto, commented, “I am delighted to have conducted the successful first in human implantation of the Topaz tricuspid valve replacement system in Canada. Implantation of the Topaz system was very easy and intuitive, and the patient, who has a complicated medical history, achieved full and sustained elimination of tricuspid regurgitation. This is a promising potential solution for patients in need.”
Prof. Dr. Treede, Director of the Department of Cardiac and Vascular Surgery at the University Medical Centre in Mainz who proctored all Topaz procedures that were performed until now, commented, “I am very pleased to have attended this implantation of the Topaz tricuspid heart valve replacement system performed by Dr. Fam and his great team at St. Michaels hospital. The smooth and successful implantation makes me even more confident that the Topaz valve represents a significant improvement in the treatment of patients with tricuspid regurgitation.”
Helmut Straubinger, CEO of TRiCares, commented, “It is a special feeling when you have developed a product and immediately after its application you can see the significant improvement of a patient’s health condition. That is what we work for. We look forward to future implantations of the Topaz system.”
About TRiCares
Founded in 2013, TRiCares is a medical device startup company headquartered in Paris, France, with its operating location in Munich, Germany. The team’s vision is to bring to the market a transfemoral tricuspid valve replacement system to help patients suffering from severe tricuspid regurgitation without the need for open heart surgery. The company is supported by leading European life science venture capital firms: Andera Partners, BioMedPartners, Credit Mutuel Innovation, GoCapital, Karista and Wellington Partners.
About Tricuspid Regurgitation (TR)
The tricuspid valve is the heart valve that regulates the blood between the right atrial and ventricular chamber. Tricuspid regurgitation occurs when the tricuspid valve fails to close properly, causing blood to flow backwards into the right atrium. Tricuspid regurgitation is a frequent problem and a severe disease that was neglected for many years, leading to a large number of untreated patients without an effective treatment option. Cardiac surgeons and interventional cardiologists have long waited for a transcatheter based solution to help patients suffering from severe TR.
About the Medical Need
Heart valve diseases are among the most serious cardiac complications affecting more than 12.7 million patients in Europe. In the last decade, innovative minimally invasive catheter-based solutions have been developed for the treatment of aortic and mitral heart valve disease, creating a fast-growing transcatheter heart valve replacement market. However, for patients with tricuspid heart valve disease (tricuspid regurgitation), no such solutions exist due to anatomic, functional and technological challenges specific to this so-called “forgotten valve”. Consequently, open-heart surgeries to repair the insufficient valve and medical treatments currently represent the standard treatment options. Due to excessive risk of the procedures (10–35 % surgical mortality), more than 99 % of TR patients are considered ineligible for the curative surgeries and are only maintained on symptomatic pharmacologic treatment with poor prognosis (2.2 years median survival). Therefore, cardiac surgeons are urgently seeking minimally-invasive, low-risk solutions to improve clinical outcomes in TR patients with no other viable treatment option.
Immatics Announces First Patient Treated with ACTengine® IMA203 TCR-T in Combination with Checkpoint Inhibitor Opdivo® (nivolumab) in Patients with Advanced Solid Tumors
Immatics Announces First Patient Treated with ACTengine® IMA203 TCR-T in Combination with Checkpoint Inhibitor Opdivo® (nivolumab) in Patients with Advanced Solid Tumors
Immatics Announces First Patient Treated with ACTengine® IMA203 TCR-T in Combination with Checkpoint Inhibitor Opdivo® (nivolumab) in Patients with Advanced Solid Tumors
- The Phase 1b dose expansion cohort will evaluate safety, biological activity and initial anti-tumor activity of IMA203 TCR-T targeting PRAME in combination with nivolumab1, a PD-1 immune checkpoint inhibitor, in patients with multiple solid tumors
- Initiation of the combination treatment follows positive interim results from the IMA203 monotherapy Phase 1a dose escalation cohort and determination of provisional recommended phase 2 dose
- IMA203 targets an HLA-A*02-presented peptide derived from the protein PRAME that is highly prevalent and homogenously expressed at high target copy numbers across several solid cancer indications
- IMA203 and nivolumab combination is part of Immatics’ strategy to realize the full clinical potential of IMA203 TCR-T targeting PRAME; initial data read-out is planned for YE 2022
Houston, Texas and Tuebingen, Germany, May 18, 2022 – Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, today announced that the first patient has been dosed in the IMA203 and nivolumab combination Phase 1b dose expansion cohort. This cohort will evaluate Immatics’ TCR-engineered cell therapy (TCR-T) approach ACTengine® IMA203 targeting an HLA-A*02-presented peptide derived from PRAME, in combination with Bristol Myers Squibb’s PD-1 checkpoint inhibitor nivolumab, in patients with advanced solid tumors. The objectives of the study will be to evaluate the safety, biological activity, and initial anti-tumor activity of the IMA203 and nivolumab combination.
“Initiating the second of three dose expansion cohorts is an important milestone in our comprehensive approach to target PRAME. It builds on the successful completion of the dose escalation part of the Phase 1 trial and the early positive clinical data we observed with IMA203,” said Cedrik Britten, Chief Medical Officer at Immatics. “We are excited to elucidate how the combination with an immune checkpoint inhibitor could enhance the potency of our engineered IMA203 T cells. We also look forward to initiating the third Phase 1b cohort with IMA203CD8, our next generation approach that additionally harnesses the power of CD4 T cells.”
The IMA203 and nivolumab combination Phase 1b dose expansion cohort is expected to enroll up to 18 patients with different types of solid tumors across 10 clinical trial sites in Germany and the U.S. Bristol Myers Squibb will provide Immatics, the study sponsor of the combination trial, with nivolumab as part of a clinical supply agreement. Nivolumab has become the standard of care treatment for many solid cancer indications and we believe it fits well into the IMA203 treatment and observation schedule. According to the clinical trial protocol for ACTengine® IMA203, nivolumab will be administered at regular intervals following IMA203 treatment. The primary endpoint of this cohort is to assess the safety of the combination. Anti-tumor activity resulting from the drug combination is a secondary endpoint, which will be assessed through imaging and measured according to the standard Response Evaluation Criteria In Solid Tumors (RECIST).
The combination treatment of IMA203 and nivolumab is part of Immatics’ strategy to realize the full clinical potential of IMA203 TCR-T targeting PRAME. Based on this strategy, the company has expanded the IMA203 trial to a total of three Phase 1b dose expansion cohorts – each designed to assess observed objective response rates, demonstrate durability of response, and form the basis for enrollment in pivotal studies. In addition to the IMA203 and nivolumab combination (first patient treated, initial data read-out planned for YE 2022), Immatics will also investigate IMA203 as monotherapy (patient enrollment ongoing, next data read-out planned in 2H 2022) and IMA203CD8, a next-generation cell therapy where IMA203-engineered T cells are co-transduced with a CD8αβ co-receptor (initiation planned for 2Q 2022, initial data read-out planned for YE 2022).
About IMA203 and target PRAME
ACTengine® IMA203 T cells are directed against an HLA-A*02-presented peptide derived from preferentially expressed antigen in melanoma (PRAME), a protein frequently expressed in a large variety of solid cancers thereby supporting the programs’ potential to address a broad cancer patient population. Immatics’ PRAME peptide is present at a high copy number per tumor cell and is homogenously and specifically expressed in tumor tissue. The peptide has been identified and characterized by Immatics’ proprietary mass spectrometry-based target discovery platform XPRESIDENT®. Through its proprietary TCR discovery and engineering platform XCEPTOR®, Immatics has generated a highly specific T cell receptor (TCR) against this target for its TCR-based cell therapy approach, ACTengine® IMA203.
About ACTengine®
ACTengine® is a personalized approach for patients with advanced solid tumors. The patient’s own T cells are genetically engineered to express a novel, proprietary TCR directed against a defined cancer target. The modified T cells are then reinfused into the patient to attack the tumor. The approach is also known as TCR-engineered cell therapy (TCR-T). All Immatics’ ACTengine® product candidates can be rapidly manufactured utilizing a proprietary manufacturing process designed to enhance T cell engraftment and persistence in vivo.
The ACTengine® T cell products are manufactured at the Evelyn H. Griffin Stem Cell Therapeutics Research Laboratory in collaboration with UTHealth. The ACTengine® Programs are co-funded by the Cancer Prevention and Research Institute of Texas (CPRIT).
– END –
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
For regular updates about Immatics, visit www.immatics.com. You can also follow us on Instagram, Twitter and LinkedIn.
Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the timing of product candidates and Immatics’ focus on partnerships to advance its strategy are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
| Media and Investor Relations Contact | |
| Jacob Verghese or Stephanie May | |
| Trophic Communications | |
| Phone: +49 89 2070 89831 | |
| Immatics N.V. | |
| Anja Heuer | Jordan Silverstein |
| Director, Corporate Communications | Head of Strategy |
| Phone: +49 89 540415-606 | Phone: +1 281 810 7545 |
Koa Health Partners with Virgin Pulse to Extend Mental Health Programs via VP+ Program
Koa Health Partners with Virgin Pulse to Extend Mental Health Programs via VP+ Program
Koa Health Partners with Virgin Pulse to Extend Mental Health Programs via VP+ Program
Koa Health, a leading global provider of digital mental healthcare solutions, today announced a new partnership with Virgin Pulse, the leading global digital-first health and wellbeing company, to provide a streamlined, cost-effective path for employers to deliver personalized mental health programs to their employees.
Koa Health is among the latest solutions available in the Virgin Pulse partner ecosystem and is a preferred partner in its VP+ partner bundle program, making it easy for organizations to connect their members with a broad range of specialized health and wellbeing programs at significant cost savings that drive lasting health outcomes and achieve high-impact business results.
“Traditional care pathways are missing the mark for the majority of employees who are showing symptoms of mental health challenges, like feeling down, having anxious thoughts, showing signs of burnout and more, but who do not have a formal diagnosis. Coupled with the shortage of mental healthcare professionals at an all-time high, business leaders need to act now to support their people to feel and perform their best at work and beyond,” said Jennifer Gendron, Chief Commercial Officer at Koa Health.
A 2021 survey of more than 45,000 people globally revealed that nearly half of people with clinical mental health challenges won’t seek help in person, citing preference for self-help, lack of confidence in treatment, affordability and stigma. In an era where mental health is a key priority across every major pillar of society, Koa Health empowers employers and health plans to quickly address this ‘missing middle’ by making mental health tools with clinical rigor easily accessible, at scale, to all of their unique populations.
The Virgin Pulse partner ecosystem is seamlessly integrated into its Homebase for Health® platform, alongside comprehensive digital capabilities and live services. The platform guides members to make the best possible decisions at every stage of their health and wellbeing journey. As a Virgin Pulse partner, Koa Health has been vetted through world-class, comprehensive privacy, security and business operations reviews. Contracting, procurement, and billing are also streamlined through the partnership.
“A company’s most valuable asset is its people. Virgin Pulse works with organizations to address the needs that matter most to them and their employees, and strives to generate strong health, wellbeing and business outcomes,” said Winston Ball, vice president, partner ecosystem at Virgin Pulse. “By accessing Koa Health through the Virgin Pulse partner ecosystem, organizations can quickly roll out a mental health solution in this critical time of need, while reducing administrative burden.”
Read how Koa Health is helping organizations around the world improve mental health for their employees. Visit www.koahealth.com or follow us at @KoaHealth on LinkedIn and Twitter to learn more.
About Koa Health
Koa Health is the leading global provider offering evidence-based, personalized, integrated solutions and services that deliver mental health for everyone. Available to more than 3 million users worldwide, Koa Health addresses a vast spectrum of mental health needs – from improving wellbeing to supporting treatment for the most prolific disorders. Backed by investors such as Telefónica, a consortium advised by Ancora Finance Group, Wellington Partners Life Sciences, and MTIP, Koa Health leverages deep clinical expertise, research and technology to deliver effective and accessible care that adapts to users’ unique circumstances, leading to lasting behavior change and positive health outcomes. Koa Health partners with employers, health plans, health systems and providers around the world with its headquarters in the Netherlands and operations in Boston, London and Barcelona.
About Koa Foundations
Koa Health’s flagship app, Koa Foundations, is ranked as the number one mental wellbeing app by expert mental health reviewers ORCHA and OneMind’s Psyberguide, boasts top user ratings across app stores, and has proven health outcomes in sleep, resilience and overall wellbeing in just two weeks. Built by leading experts in neuroscience, psychiatry, behavioral therapy and digital therapeutics, Koa Health’s solutions leverage evidence-based programs such as cognitive-behavioral therapy (CBT), positive psychology, mindfulness, meditation, relaxation techniques, psychoeducation, emotional regulation, and acceptance and commitment training (ACT) to drive health outcomes and deliver long-lasting behavior change. Powered by a proprietary AI recommendation engine, employees have access to a rich library of exclusive, bite-sized educational content, tools, interventions, activities and games designed to provide an engaging employee benefit for a diverse population.
Immatics Initiates Phase 1 Clinical Trial to Evaluate Lead TCR Bispecific IMA401 in Patients with Advanced Solid Tumors
Immatics Initiates Phase 1 Clinical Trial to Evaluate Lead TCR Bispecific IMA401 in Patients with Advanced Solid Tumors
Immatics Initiates Phase 1 Clinical Trial to Evaluate Lead TCR Bispecific IMA401 in Patients with Advanced Solid Tumors
- Patient enrollment for IMA401 Phase 1 trial started at first clinical site in Germany
- The study will evaluate safety, tolerability, and initial anti-tumor activity of IMA401 in patients with recurrent and/or refractory solid tumors
- TCER® IMA401 targets MAGEA4/8 and will be developed in collaboration with Bristol Myers Squibb
Tuebingen, Germany and Houston, Texas, May 10, 2022 – Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell-redirecting cancer immunotherapies, today announced the initiation of a Phase 1 clinical trial with its T cell engaging receptor (TCER®) IMA401 for patients with recurrent and/or refractory solid tumors. IMA401 is the most advanced product candidate from Immatics’ TCR Bispecific pipeline targeting an HLA-A*02-presented peptide derived from both MAGEA4 and MAGEA8. TCER® IMA401 will be developed in collaboration with Bristol Myers Squibb. Immatics is responsible for conducting the Phase 1 clinical trial.
The primary objectives of the clinical trial (NCT#05359445) are to determine the maximum tolerated dose (MTD) and/or the recommended phase 2 dose (RP2D) for IMA401 in biomarker-positive (HLA-A*02:01 and MAGEA4/8) patients with recurrent and/or refractory solid tumors. Secondary objectives are to characterize safety and tolerability, evaluate initial anti-tumor activity and assess pharmacokinetics of IMA401. The Phase 1 trial consists of a dose-escalation (Phase 1a) portion that will be followed by a dose-expansion (Phase 1b) portion to treat patients at the recommended dose level. The trial is planned to be conducted at up to 15 centers in Germany, with the first site already being initiated. The Phase 1 trial is designed to enroll approximately 50 patients.
“IMA401 is the first TCER® candidate from our TCR Bispecifics pipeline entering clinical development, and expands our clinical portfolio with an exciting new TCR-based immunotherapy approach that can be supplied off-the-shelf compared to autologous cell therapies,” said Cedrik Britten, Chief Medical Officer at Immatics. “Our innovative TCER® format leads to an extended-half-life and incorporates novel binding-moieties that are designed to maximize efficacy while minimizing toxicities in patients. Our TCER® IMA401 could treat a range of solid tumors and therefore meet currently unmet needs of a broad patient population. This is best achieved with a strong pharma partner which we have found in Bristol Myers Squibb.”
Immatics entered into a global exclusive license agreement with Bristol Myers Squibb in December 2021 for the IMA401 program under which both companies will collaborate to advance the program through clinical development.
Immatics’ TCR Bispecific pipeline includes a second TCER® product candidate, IMA402, which targets PRAME. Manufacturing of the clinical IMA402 batch is planned for the second half of 2022 and initiation of the Phase 1 trial is planned in 2023. Immatics’ TCER® pipeline is further strengthened by additional innovative TCER® program(s), IMA40X, in preclinical development.
About IMA401
IMA401 is Immatics’ most advanced TCER® molecule that targets an HLA-A*02-presented (human leukocyte antigen) peptide derived from two different cancer-associated proteins, melanoma-associated antigen 4 and/or 8 (“MAGEA4/8”). The MAGEA4/8 peptide has been identified and validated by Immatics’ proprietary mass spectrometry-based target discovery platform XPRESIDENT® and is presented at a 5-fold higher copy number per tumor cell than a MAGEA4 peptide targeted in other clinical trials. Following preclinical proof-of-concept data, including complete remissions of transplanted human-derived tumors in xenograft mouse models, the Phase 1 trial investigates IMA401 in patients with tumors of high MAGEA4/8 prevalence, such as squamous non-small cell lung carcinoma (sqNSCLC), small cell lung cancer (SCLC), head and neck squamous cell carcinoma (HNSCC), bladder, uterine, esophageal and ovarian carcinomas, as well as melanoma, sarcoma subtypes and other solid cancer types.
About TCER®
Immatics’ half-life extended TCER® molecules are antibody-like “off-the-shelf” biologics that leverage the body’s immune system by redirecting and activating T cells towards cancer cells expressing a specific tumor target. The design of the TCER® molecules enables the activation of any T cell in the body to attack the tumor, regardless of the T cells’ intrinsic specificity. Immatics proprietary biologics are engineered with two binding regions: a TCR domain and a T cell recruiter domain. The TCER® format is designed to maximize efficacy while minimizing toxicities in patients. It contains a high-affinity TCR domain that is designed to bind specifically to the cancer target peptide on the cell surface presented by an HLA molecule. The antibody-derived, low-affinity T cell recruiter domain is directed against the TCR/CD3 complex and recruits a patient’s T cells to the tumor to attack the cancer cells. With a low-affinity recruiter aiming for optimized biodistribution and enrichment of the molecule at the tumor site instead of the periphery, TCER® are engineered to reduce the occurrence of immune-related adverse events, such as cytokine release syndrome. In addition, the TCER® format consists of an Fc-part conferring half-life extension, stability, and manufacturability. TCER® are “off-the-shelf” biologics and thus immediately available for patient treatment. They can be distributed through standard pharmaceutical supply chains and provide the opportunity to reach a large patient population without the need of specialized medical centers.
– END –
About Immatics
Immatics combines the discovery of true targets for cancer immunotherapies with the development of the right T cell receptors with the goal of enabling a robust and specific T cell response against these targets. This deep know-how is the foundation for our pipeline of Adoptive Cell Therapies and TCR Bispecifics as well as our partnerships with global leaders in the pharmaceutical industry. We are committed to delivering the power of T cells and to unlocking new avenues for patients in their fight against cancer.
For regular updates about Immatics, visit www.immatics.com. You can also follow us on Twitter, Instagram and LinkedIn.
Forward-Looking Statements:
Certain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or Immatics’ future financial or operating performance. For example, statements concerning the timing of product candidates and Immatics’ focus on partnerships to advance its strategy are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, uncertainties and factors set forth in filings with the SEC. Nothing in this presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Immatics undertakes no duty to update these forward-looking statements. All the scientific and clinical data presented within this press release are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
For more information, please contact:
| Media and Investor Relations Contact | |
| Jacob Verghese or Stephanie May | |
| Trophic Communications | |
| Phone: +49 89 2070 89831 | |
| Immatics N.V. | |
| Anja Heuer | Jordan Silverstein |
| Director, Corporate Communications | Head of Strategy |
| Phone: +49 89 540415-606 | Phone: +1 281 810 7545 |
UroMems Appoints Steffen Hovard to Chairman of the Board of Directors
UroMems Appoints Steffen Hovard to Chairman of the Board of Directors
U.S.-based medical device industry leader’s objective will be to increase U.S. financial, clinical development and commercial footprint
GRENOBLE, France, May 11, 2022 (GLOBE NEWSWIRE) — UroMems, developer and manufacturer of UroActive™, the first active medical implantable device for treating stress urinary incontinence, today announced the elevation of Steffen Hovard, currently on UroMems’ Board, to Chairman of the Company’s Board of Directors. Mr. Hovard is a medical device industry leader in the U.S, with a successful track record in urology. As chairman, he will be focused on increasing UroMems’ financial, clinical development and commercial footprint in the U.S.
“I joined UroMem’s Board in 2020 because I believed that the UroActive™ device is truly innovative and potentially transformational treatment for the millions of men and women worldwide who suffer from stress urinary incontinence or SUI,” said Mr. Hovard. “Now as we approach the initiation of clinical trials in the U.S., I was offered the opportunity to increase my involvement with UroMems by taking on the responsibility as Chairman of the Board. I hope to further assist the Company in reaching its goals of increasing U.S. investors’ interest, launching clinical trials and anticipating the commercial launch in the U.S. and other geographies.”
For more than 20 years, Mr. Hovard has been a prominent leader in the medical device industry especially in urology. He has held multiple leadership roles at subsidiaries of Coloplast, a publicly traded global medical device company. Most recently, from 2011 to 2019, he served as the President of the global Interventional Urology business, a global $300M fast growing business with a broad portfolio of both single use and implantable devices. Mr. Hovard currently serves on a number of medical device company Boards. Having worked internationally throughout his career, and now living in the U.S., Mr. Hovard brings a geographical and business perspective to UroMems, giving a solid foundation for the company’s transformation to the next stage, as it prepares for clinical trial and eventually commercialization.
“The U.S. is an important geography for UroMems because a large percentage of urological procedures occur there,” said Hamid Lamraoui, Chief Executive Officer and co-founder of UroMems. “This is also the opportunity for the company to bring on board of U.S.-based investors involved in emerging innovative medical device companies. In addition, Steffen has been a key contributor to the Company’s development since he joined our Board in 2020. We are grateful that he has accepted the additional responsibility as Chairman of our Board of Directors. His strong reputation within the medical device industry will be of great assistance as we transition from being a French emerging medical device company into a global, clinical stage medical device company.”
About UroActive™
UroActive™ is an active implantable electronic artificial urinary sphincter that compensates for sphincter insufficiency in patients, both men and women, with SUI. It is based on a unique mechatronic platform using embedded smart, AI-based, digital & robotic systems which, based on data collected from a patient, creates a treatment algorithm that is specific for each patient’s needs. The UroMem’s technology platform is protected by more than 100 patents and is designed to overcome the limitations of current solutions by optimizing safety and performance, patient experience and surgeon convenience.
About UroMems
Founded in 2011 by Pr Pierre Mozer, Hamid Lamraoui and Stéphane Lavallée, UroMems aims to restore the quality of life, dignity and self-esteem of millions of men and women worldwide suffering from untreated chronic conditions by the commitment to change the perception that these disorders are inevitable as one grows older and is simply something to endure with no real solution. The first challenge for the company will be applying robotic methods and smart systems for treating urinary incontinence: designed by urologists and collaborating scientists and engineers, UroActive™ will present a new standard of care combining safety, efficacy, durability and ergonomics fitting any individual’s lifestyle and anatomy.
Since the inception of the company, significant investments have been made for the development of UroMems’ first product. This includes 2 financing rounds totaling $30 million, led by Wellington Partners, Bpifrance, Supernova Invest, Cita Investissement, btov Partners, Hil-Invent and Financière Arbevel. The company has been awarded by several renowned innovation prizes including the Prix Galien Award Medstart’up, and the Worldwide Innovation Challenge initiated by the French government. For more information, please visit www.uromems.com
Media: Robert Flamm
Burns McClellan
Digital Therapeutics Leader Sidekick Raises $55M Series B To Drive Further Growth
Digital Therapeutics Leader Sidekick Raises $55M Series B To Drive Further Growth
- The closing of the company’s B Series follows an oversubscribed $20m A round in 2020.
- The funding allows Sidekick to accelerate the multi-chronic capabilities of its therapeutics platform.
- The company is accelerating its growing commercial footprint in the United States.
BERLIN, BOSTON, REYKJAVIK, 05 May 2022 – International digital therapeutics leader Sidekick Health (www.sidekickhealth.com) today announces that it has raised $55 million in Series B funding. The round was led by London-based venture capital firm Novator Ventures, with additional participation from Wellington Partners, Asabys Partners, and Frumtak Ventures, as well as a US-based strategic investor that will be revealed at a later stage.
Sidekick is on a mission to develop and deliver effective, personalized digital therapeutics that prevent suffering and add millions of quality life years to people around the world. Digital therapeutics are the next generation of therapeutics designed to prevent, treat and help people manage a wide range of medical disorders or diseases.
“By harnessing the power of technology we have a unique opportunity to deliver personalized treatments via smartphones and other mobile devices, empowering people to take control of their own health,” Sidekick’s chief executive officer and co-founder Dr. Tryggvi Thorgeirsson said.
“The major challenge facing the world’s healthcare systems is how to support and treat people who are dealing with with two or more chronic conditions. The cornerstone of Sidekick’s approach is our multi-chronic digital therapeutics platform, and this funding will allow us to scale even faster the production of a new generation of clinical treatments across all major chronic diseases,” Thorgeirsson added.
Novator Ventures general partner and founder Birgir Mar Ragnarsson joins Sidekick’s board of directors in conjunction with the closing of the Series B financing.
“It has been impressive to follow the rapid growth of the company from the close of its A round 18 months ago. The company may have begun in the Nordics, but I am proud to say that Sidekick is now a globally-recognized digital therapeutics player,” Ragnarsson said. “Novator Ventures recognizes the immense opportunities presented by third generation therapeutics and Sidekick’s ability to scale and operate at the global level. We look forward to working closely with the Sidekick team to transform how healthcare is delivered.”
Sidekick operates its platform in partnership with some of the biggest names in healthcare, including leading US-based health company Anthem Inc. to offer a digital-first care programs, as well as global pharmaceutical majors such as Pfizer and Bayer, to develop integrated combination therapeutics consisting of a molecular drug and a digital therapeutic.
In 2019, Sidekick entered into a strategic partnership with Bayer to provide a digital therapeutic to patients with Peripheral Artery Disease (PAD). In 2020, Sidekick then secured a deal with Pfizer addressing five inflammatory diseases across Europe and beyond.
Following this, during the height of the pandemic, Sidekick assisted the Government of Iceland by providing the remote triaging, remote monitoring and management of people infected with COVID-19. Shortly after, the company raised a $20 million Series A led by Wellington Partners and Asabys Partners. Over the past year Sidekick has further
increased its commercial footprint in the US, including a collaboration with Anthem to support members dealing with COVID-19, Crohn’s disease, and other chronic conditions.
Amidst this backdrop of this rapid growth, the company is now looking to further diversify its portfolio by expanding its chronic disease treatments across even more therapeutic areas, bolstering existing partnerships and forging new alliances with key stakeholders across the healthcare ecosystem.
About Sidekick Health
Sidekick is a digital therapeutics innovator, founded by two passionate medical doctors on a mission to improve the health of humanity. Sidekick is driven by a vision to bring healthcare into people’s everyday lives, empowering them to take control of their own health. Sidekick’s therapeutics are developed and delivered with digital technology, addressing the same endpoints as traditional treatments – such as small molecule drugs and biologics, but with the added benefit of giving people the opportunity to be actively involved in their own treatment. When combined with traditional treatments, the overall efficacy can be strongly increased. That is why Sidekick partners with the world’s most innovative companies across the healthcare spectrum. Sidekick has offices in Berlin, Boston, Reykjavik and Stockholm.
www.sidekickhealth.com
For media inquiries
Please contact
Sidekick
Gulli Arnason, CMCO
+354 660 0053
Sam Hearne
0044 (0)7308 889 791
TRiCares Announces Successful First in Human Implantation of Minimally Invasive Topaz Tricuspid Heart Valve Replacement System in Germany
TRiCares Announces Successful First in Human Implantation of Minimally Invasive Topaz Tricuspid Heart Valve Replacement System in Germany
Paris, France and Munich, Germany, April 25, 2022 – TRiCares SAS (“TRiCares”) a privately held pioneer in the field of minimally invasive treatment of tricuspid regurgitation, today is pleased to announce the successful first in human implantation of its Topaz transfemoral tricuspid heart valve replacement system (“Topaz”) in Germany.
Heart valve diseases are among the most serious cardiac conditions, affecting more than 12.7 million patients in Europe and many more worldwide. In the last decade minimally invasive catheter-based solutions have been developed for other heart valve diseases, but none have been designed specifically for the tricuspid valve.
Tricuspid regurgitation is a frequent and serious disease for which open heart surgery and symptomatic pharmacologic treatment are the current standard treatment options. Owing to high mortality risk, access to open heart surgery is severely restricted and is not considered an option for more than 99 % of patients with tricuspid regurgitation. The prognosis for patients without surgical repair is poor, with 2.2 years median survival. As such, there is an urgent need for minimally invasive, lower risk solutions to improve outcomes for patients with no other viable treatment options.
Topaz is an innovative device designed specifically to help patients suffering from severe tricuspid regurgitation without the need for open heart surgery. The Topaz device is the result of a French and German collaboration and is implanted in a minimally invasive procedure through the patient’s femoral vein. It is designed specifically to fit the tricuspid valve anatomy and thus supports ease of positioning and functionality.
Today’s announcement marks the successful first in human implantation of Topaz in a patient in Germany, which was done as a compassionate use treatment.
The procedure in Germany was performed for a 76-year-old woman with heart failure due to massive tricuspid regurgitation that recurred despite a previous suture annuloplasty of the tricuspid valve in 2014. The patient has a history of multiple cardiac surgeries like mitral valve replacement and bypass surgery, and has numerous risk factors that made a surgical treatment too hazardous. The successful implantation of the Topaz tricuspid heart valve replacement system took place at University Hospital Mainz in Germany, on 8 April 2022, and was performed by Prof. Ralph Stephan von Bardeleben. Prof. Hendrik Treede, cardiac surgeon at University Hospital Mainz, and Prof. Ulrich Schäfer, interventional cardiologist of the military hospital in Koblenz, Germany proctored the procedure. After an implantation time of 25 minutes the Topaz prosthesis anchored safely in the significantly enlarged ventricle and achieved complete correction of the tricuspid regurgitation. The patient recovered quickly from the intervention and was discharged from hospital after eight days.
In total nine implantations of the Topaz tricuspid heart valve replacement system have been performed to date across Europe.
Building upon the success of these procedures, TRiCares is preparing a clinical study in the coming months to confirm the value of its Topaz tricuspid heart valve replacement system for these types of patients, who until now have had no satisfactory treatment option.
Prof. Dr. Ralph Stephan von Bardeleben, Head of Heart Valve Center Mainz at University Hospital Mainz, commented, “I am delighted to have conducted the first implantation of the Topaz tricuspid valve replacement system in Germany. This patient, like the vast majority of patients with tricuspid regurgitation, had no other viable treatment options. It is tremendous to have successfully treated her using the innovative Topaz system, which provided perfect seating and valve functionality, and I hope this will be available in future for all patients in need.”
Prof. Dr. Treede, Director of the Department of Cardiac and Vascular Surgery at the University Medical Centre in Mainz, who proctored all Topaz procedures that were performed until now, commented, “I am very pleased to have attended all of the implantations of the Topaz tricuspid heart valve replacement system, which represents a significant improvement in the treatment of patients with tricuspid regurgitation.”
Helmut Straubinger, CEO of TRiCares, commented, “I am very glad to announce the first successful implantation of our Topaz tricuspid heart valve replacement system in Germany. I am proud of our team for developing an innovative treatment possibility for severely ill patients suffering from tricuspid regurgitation with no other treatment options. This successful implantation together with the results of further eight compassionate use cases give us confidence in our development and in taking the next steps.”
About TRiCares
Founded in 2013, TRiCares is a medical device startup company headquartered in Paris, France, with its operating location in Munich, Germany. The team’s vision is to bring to the market a transfemoral tricuspid valve replacement system to help patients suffering from severe tricuspid regurgitation without the need for open heart surgery. The company is supported by leading European life science venture capital firms: Andera Partners, BioMedPartners, Credit Mutuel Innovation, GoCapital, Karista and Wellington Partners.
About Tricuspid Regurgitation (TR)
The tricuspid valve is the heart valve that regulates the blood between the right atrial and ventricular chamber. Tricuspid regurgitation occurs when the tricuspid valve fails to close properly, causing blood to flow backwards into the right atrium. Tricuspid regurgitation is a frequent problem and a severe disease that was neglected for many years, leading to a large number of untreated patients without an effective treatment option. Cardiac surgeons and interventional cardiologists have long waited for a transcatheter based solution to help patients suffering from severe TR.
About the Medical Need
Heart valve diseases are among the most serious cardiac complications affecting more than 12.7 million patients in Europe. In the last decade, innovative minimally invasive catheter-based solutions have been developed for the treatment of aortic and mitral heart valve disease, creating a fast-growing transcatheter heart valve replacement market. However, for patients with tricuspid heart valve disease (tricuspid regurgitation), no such solutions exist due to anatomic, functional and technological challenges specific to this so-called “forgotten valve”. Consequently, open-heart surgeries to repair the insufficient valve and medical treatments currently represent the standard treatment options. Due to excessive risk of the procedures (10–35 % surgical mortality), more than 99 % of TR patients are considered ineligible for the curative surgeries and are only maintained on symptomatic pharmacologic treatment with poor prognosis (2.2 years median survival). Therefore, cardiac surgeons are urgently seeking minimally-invasive, low-risk solutions to improve clinical outcomes in TR patients with no other viable treatment option.
For further information please contact:
TRiCares SAS
Helmut J. Straubinger,
President and
Chief Executive Officer
Consilium Strategic Communications
Matthew Cole
T: +44 (0)20 3709 5700
eGenesis Promotes Michael Curtis, Ph.D., to Chief Executive Officer
eGenesis Promotes Michael Curtis, Ph.D., to Chief Executive Officer
eGenesis Promotes Michael Curtis, Ph.D., to Chief Executive Officer
Dr. Curtis succeeds Paul Sekhri, who remains on Board of Directors.
Reflects company’s focus on advancement of portfolio toward clinical development.
CAMBRIDGE, Mass., April 19, 2022 – eGenesis, a biotechnology company developing human-compatible organs and cells for the treatment of organ failure, today announced the promotion of Michael Curtis, Ph.D., to Chief Executive Officer (CEO). Dr. Curtis succeeds Paul Sekhri who remains on the Board of Directors.
Dr. Curtis has served as President of Research & Development at eGenesis since joining the Company in 2020. He brings to eGenesis more than 25 years of scientific research and leadership experience in biopharmaceuticals across numerous companies and therapeutic areas. Dr. Curtis joined eGenesis from Cadent Therapeutics, where he served as President and Head of R&D. During his tenure at Cadent, the company developed a pipeline of novel therapies for the treatment of psychiatric and movement disorders leading to its acquisition by Novartis at the end of 2020.
“It has been a privilege to manage our research and development efforts under Paul’s leadership these past eighteen months,” Dr. Curtis said, “The team is focused on our mission of solving the organ shortage crisis. I am excited to continue advancing that mission and I am grateful for the confidence shown in me by the Board and my colleagues at eGenesis.”
Paul Sekhri former President and Chief Executive Officer and current Board member of eGenesis said, “I have seen Mike accelerate our engineering, production and preclinical development efforts during his tenure at eGenesis, and I am confident that he is the leader to advance our programs toward the clinic. I look forward to continue working with Mike as a member of the Board as we work toward the day when no one dies while waiting for a transplant.”
The timing of the transition coincides with the company’s efforts to advance multiple programs toward clinical development. The company recently appointed Dr. Eliezer Katz as Chief Medical Officer of eGenesis to lead medical operations and support preparation of programs for the clinic.
Outgoing CEO Paul Sekhri has led eGenesis since 2019, overseeing efforts that brought in $225 million for the company in support of the development of its platform, an acquisition in 2020 to vertically integrate donor production, and the expansion of the eGenesis team and operations.
“Paul has been critical in developing eGenesis into the company we are today,” said Steven Gillis, Ph.D., Chairman of the Board of Directors of eGenesis. “After more than 25 years as a biotechnology industry leader, Paul will focus on providing guidance to multiple life science companies. Mike has been successfully advancing our R&D programs since joining the Company in 2020. His elevation to the CEO role is a natural step for him and provides leadership continuity at eGenesis. The Board, management and the rest of the team are excited about his promotion and look forward to his leadership as we advance our programs toward clinical development.”
About Transplantation and Xenotransplantation
The demand for lifesaving organs far outnumbers available supply. In the U.S. alone, more than 100,000 people are on the national transplant list. Twenty people die every day due to lack of available organs for transplant and every 10 minutes, a new name is added to the national transplant waitlist.
The concept of xenotransplantation (the transplantation of organs and cells from one species to another) has been explored for decades. However, virology and immunology hurdles prevented the field from advancing beyond early preclinical research. With the advent of cutting-edge gene editing technologies, addressing these historical challenges is now within reach.
About eGenesis
eGenesis’ goal is to transform the field of transplantation by offering safe and effective organs and cells to patients in need. The company harnesses gene editing technology including CRISPR to address the key issues that have impeded xenotransplantation to date. eGenesis’ development pipeline includes lead programs for kidney and islet cell transplant as well as earlier-stage programs focused on other solid organs. Learn more at eGenesisbio.com.
Contacts:
Investors/Media
Investors
Eric Ando / Dr. Grace Kim
Burns McClellan, Inc.
212-213-0006
/
Media
Robert Flamm, Ph.D. / Katie Larch
Burns McClellan, Inc.
212-213-0006 ext. 364
/
ONWARD Announces Additions to Leadership Team
ONWARD Announces Additions to Leadership Team
Lara Smith Weber to join the company as CFO and Zouhir Mechta to join as VP Operations.
EINDHOVEN, the Netherlands & LAUSANNE, Switzerland—April 13, 2022–ONWARD Medical N.V. (Euronext: ONWD), the medical technology company creating innovative therapies to restore movement, independence, and health in people with spinal cord injury, today announces Lara Smith Weber will join the company as its Chief Financial Officer and Zouhir Mechta will join the company as its Vice President Operations
PEI Regulatory Approval Received for Government Funded (€ 7.4 Million) Clinical Trial Investigating Adrenomed’s Adrecizumab in Treating Endothelial Barrier Dysfunction in Hospitalized COVID-19 Patients
PEI Regulatory Approval Received for Government Funded (€ 7.4 Million) Clinical Trial Investigating Adrenomed’s Adrecizumab in Treating Endothelial Barrier Dysfunction in Hospitalized COVID-19 Patients
PEI Regulatory Approval Received for Government Funded (€ 7.4 Million) Clinical Trial Investigating Adrenomed’s Adrecizumab in Treating Endothelial Barrier Dysfunction in Hospitalized COVID-19 Patients
- Adrecizumab selected to receive funding from two COVID-19 drug development programs of the German Federal Ministry of Education and Research (BMBF)
- By restoring vascular integrity, Adrecizumab addresses the endothelial barrier dysfunction as a consequence of severe COVID-19
- University Medical Center Eppendorf (UKE) in Hamburg initiates Phase II trial to evaluate precision medicine approach with Adrecizumab to treat hospitalized patients with moderate to severe COVID-19
Hennigsdorf/Berlin (Germany), April 11, 2022 – Adrenomed AG, the vascular integrity company, today announced that its proprietary sepsis drug candidate Adrecizumab (HAM8101) received a funding commitment of € 7.4 million from the German Federal Ministry of Education and Research (BMBF) as part of the German funding initiatives for the clinical development of COVID-19 drugs and their manufacturing capabilities.[1],[2] The University Medical Center Eppendorf (UKE) Hamburg and Adrenomed will receive funds to conduct an investigator initiated Phase II clinical trial with Adrecizumab in hospitalized patients with moderate to severe COVID-19, which has received regulatory approval by the German federal agency, Paul Ehrlich Institute (PEI). Additionally, the BMBF will support financially the manufacturing of GMP Phase III clinical trial material.
Adrecizumab, a first-in-class antibody targeting the vasoprotective peptide adrenomedullin, is currently being developed for the treatment of loss of vascular integrity in sepsis and septic shock. Dysregulation of the endothelial barrier appears to be a common feature of COVID-19 patients and sepsis or septic shock patients. The deteriorated endothelial barrier function leads to vascular leakage and severe impairment of lung and other organ functions. In a novel precision medicine approach, patients with elevated adrenomedullin levels will be treated with Adrecizumab to restore and maintain the endothelial barrier and to avoid further organ dysfunction. At the same time, another biomarker (dipeptidyl peptidase-3, DPP3) is used to exclude patients with an additional competing pathophysiology. The UKE has initiated a national, multicenter, biomarker-guided, placebo-controlled Phase II trial to evaluate the efficacy of Adrecizumab plus standard of care (SOC) and plans to enroll more than 200 patients with moderate to severe COVID-19 and elevated adrenomedullin levels.[3]
Prof. Dr. med. Stefan Kluge, Director of the Department of Intensive Care Medicine at UKE, said: “We urgently need effective drugs for the treatment of hospitalized COVID-19 patients. Particularly important are therapies that can be used in severe cases and can be given at later disease stages. Adrecizumab could potentially be a therapy that can address this treatment gap. In a previous named-patient program with critically ill COVID-19 patients treated with Adrecizumab, we have already observed a rapid improvement in organ function. Based on these results and the positive signals from the AdrenOSS-2 study in septic shock, we will now evaluate a precision medicine approach with Adrecizumab to treat COVID-19 patients with deteriorating endothelial function and increased risk of organ failure and mortality.”
“With Adrecizumab we provide a treatment option that aims at restoring and maintaining the impaired vascular integrity in COVID-19 other than antiviral drugs”, Dr. Jens Zimmermann, Chief Medical Officer at Adrenomed added. “This approach may help to improve the organ function and thus survival rates of hospitalized COVID-19 patients. We are very grateful for this financial support granted by the BMBF which enables Adrenomed to pursue the clinical development of Adrecizumab in the treatment of COVID-19, as well as the manufacturing process.”
Dr. Richard Jones, Chief Executive Officer of Adrenomed, stated: “This funding commitment of € 7.4 million from the BMBF is a significant endorsement of Adrenomed’s innovative bio-marker guided precision medicine approach for patients in the acute care setting. This clinical study further compliments our overall development program of Adrecizumab for the treatment of loss of vascular integrity in sepsis and septic shock in collaboration with our strategic partner, Sphingotec.”
About Adrenomed
Adrenomed AG is a German privately financed, clinical-stage biopharmaceutical company. Adrenomed’s mission is to rescue vascular integrity and save the lives of critically ill patients with limited treatment options. Founded in 2009 by a management team with decades of in-depth experience in sepsis and deep knowledge in diagnostics and drug development, the company’s lead product candidate Adrecizumab (INN: enibarcimab) is a first-in-class monoclonal antibody. Adrecizumab targets the vasoprotective peptide Adrenomedullin, an essential regulator of vascular integrity. Adrecizumab has successfully completed a biomarker-guided, double-blinded, placebo-controlled, randomized, multicenter proof-of-concept Phase II trial with 301 patients suffering from septic shock. For further information, please visit www.adrenomed.com and follow us on LinkedIn and Twitter.
Contact
Adrenomed AG
Frauke Hein, Ph.D. (Chief Business Officer)
phone: +49 (0)3302 2077814
Media Inquiries
MC Services AG
Eva Bauer / Julia von Hummel
phone: +49 (0)89 21022880
[1] https://www.gesundheitsforschung-bmbf.de/de/12638.php
[2] https://www.gesundheitsforschung-bmbf.de/de/13173.php
[3] https://clinicaltrials.gov/ct2/show/NCT05156671?term=adrecizumab&draw=2&rank=4